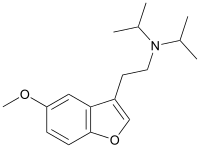
5-MeO-DiBF
5-MeO-DiBF is a psychedelic[1] that has been sold online as a designer drug and was first definitively identified in December 2015 by a forensic laboratory in Slovenia.[2] It is thought to act as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DiPT, but with the indole nitrogen replaced by oxygen, making 5-MeO-DiBF a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DiPT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors.[3][4] LEGAL STATUS. It is not controlled under the 1971 Convention on Psychotropic Substances, so thus it has a legal grey area in many countries of the world, but its consumption still could be persecuted under severe analogue acts or the intend of sell to human consumption.
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H25NO2 |
| Molar mass | 275.392 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Casale JF, Hays PA. "The Characterization of 2-(5-Methoxy-1-benzofuran-3-yl)-N,N-dimethylethanamine (5-MeO-BFE) and Differentiation from its N-Ethyl Analog" (PDF). Microgram Journal. 9 (1): 39–45.
- "Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA" (PDF). European Monitoring Center for Drugs and Drug Addiction.
- Tomaszewski Z, Johnson MP, Huang X, Nichols DE (May 1992). "Benzofuran bioisosteres of hallucinogenic tryptamines". Journal of Medicinal Chemistry. 35 (11): 2061–4. doi:10.1021/jm00089a017. PMID 1534585.
- McKenna DJ, Repke DB, Lo L, Peroutka SJ (March 1990). "Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes". Neuropharmacology. 29 (3): 193–8. doi:10.1016/0028-3908(90)90001-8. PMID 2139186. S2CID 24188017.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||