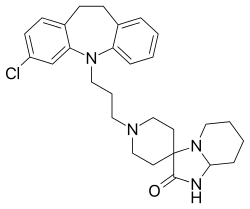
Mosapramine
Mosapramine (Cremin) is an atypical antipsychotic used in Japan for the treatment of schizophrenia.[1][2] It is a potent dopamine antagonist with high affinity to the D2, D3, and D4 receptors,[3] and with moderate affinity for the 5-HT2 receptors.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cremin (クレミン, JP) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets, oral solution) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H35ClN4O |
| Molar mass | 479.07 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
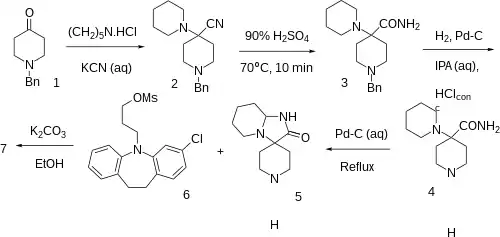
Synthesis
Note that if the ring cylization step was missed you instead get Clocapramine.
The Strecker like condensation between 1-Benzyl-4-piperidone [3612-20-2] (1), piperidine, and potasssium cyanide gives 1-benzyl-4-piperidin-1-ylpiperidine-4-carbonitrile [84254-97-7] (2). Partial hydrolysis of the nitrile to the amide gives 1-benzyl-4-piperidin-1-ylpiperidine-4-carboxamide [1762-50-1] (3). Catalytic hydrogenation then removes the benzyl protecting group to give 4-piperidin-1-ylpiperidine-4-carboxamide [39633-82-4] (4). Treatment with a palladium on carbon catalyt apparently forms spiro[1,5,6,7,8,8a-hexahydroimidazo[1,2-a]pyridine-3,4'-piperidine]-2-one, CID:13002712 (5).
Convergent synthesis with 3-(2-chloro-5,6-dihydrobenzo[b][1]benzazepin-11-yl)propyl methanesulfonate, CID:20533966 (6) completed the synthesis of Mosapramine (7).
See also
- Carpipramine
- Clocapramine
- Fluspirilene (typical antipsychotic)
- Imidazopyridine
References
- Takahashi N, Terao T, Oga T, Okada M (1999). "Comparison of risperidone and mosapramine addition to neuroleptic treatment in chronic schizophrenia". Neuropsychobiology. 39 (2): 81–5. doi:10.1159/000026565. PMID 10072664. S2CID 6554048.
- Miyamoto S (2010). "Mosapramine". In Stolerman IP (ed.). Encyclopedia of Psychopharmacology. Berlin, Heidelberg: Springer. p. 76. doi:10.1007/978-3-540-68706-1_1839. ISBN 978-3-540-68706-1. Retrieved 21 March 2022.
- Futamura T, Ohashi Y, Yano K, Takahashi Y, Haga K, Fukuda T (May 1996). "[The affinities of mosapramine for the dopamine receptor subtypes in human cell lines expressing D2, D3 and D4 receptors]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 107 (5): 247–53. doi:10.1254/fpj.107.247. PMID 8690306.
- Sumiyoshi T, Suzuki K, Sakamoto H, Yamaguchi N, Mori H, Shiba K, Yokogawa K (February 1995). "Atypicality of several antipsychotics on the basis of in vivo dopamine-D2 and serotonin-5HT2 receptor occupancy". Neuropsychopharmacology. 12 (1): 57–64. doi:10.1016/0893-133X(94)00064-7. PMID 7766287.
- Chiaki Tashiro & Ichiro Horii, EP 0073845 (1985 to Welfide Corp).
- Chiaki Tashiro & Ichiro Horii, U.S. Patent 4,337,260 (1982 to Mitsubishi Pharma Corp).
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|