Tretoquinol
Tretoquinol is a beta-adrenergic agonist.[1][2]
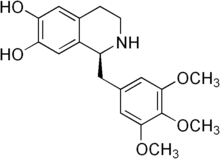 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1S)-1-[(3,4,5-Trimethoxyphenyl)methyl]-1,2,3,4-tetrahydroisoquinoline-6,7-diol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Tretoquinol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C19H23NO5 |
| Molar mass | 345.39 g/mol |
| Pharmacology | |
| R03AC09 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- "Synthesis of 6,7-dihydrox-1,2,3,4-tetrahydroisoquinoline derivatives". Tetrahedron. 22: 129–134. doi:10.1016/S0040-4020(01)82177-3.
- "β-Adrenoceptor Subtype Activities of Trimetoquinol Derivatives: Biochemical Studies on Human β-Adrenoceptors Expressed in Chinese Hamster Ovary Cells". Jpet.aspetjournals.org. 1999-11-01. Retrieved 2012-08-20.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.