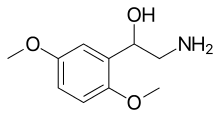
Midodrine
Midodrine is a vasopressor/antihypotensive agent (it raises the blood pressure). Midodrine was approved in the United States by the Food and Drug Administration (FDA) in 1996 for the treatment of dysautonomia and orthostatic hypotension. In August 2010, the FDA proposed withdrawing this approval because the manufacturer, Shire plc, failed to complete required studies after the medicine reached the market.[2][3] In September 2010, the FDA reversed its decision to remove midodrine from the market and allowed it to remain available to patients while Shire plc collected further data regarding the efficacy and safety of the drug.[4] Shire announced on September 22, 2011, that it was withdrawing completely from supplying midodrine and leaving it to several generics to supply the drug.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Amatine, Proamatine, Gutron, others |
| Other names | 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxy-ethyl]-acetamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602023 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.151.349 100.050.842, 100.151.349 |
| Chemical and physical data | |
| Formula | C12H18N2O4 |
| Molar mass | 254.286 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Medical uses
Midodrine is indicated for the treatment of symptomatic orthostatic hypotension. It can reduce dizziness and faints by about a third, but can be limited by troublesome goose bumps, skin itch, gastrointestinal discomfort, chills, elevated blood pressure while lying down, and urinary retention.[6] A meta-analysis of clinical trials of midodrine or droxidopa in patients with low blood pressure when standing found that midodrine increased standing blood pressure more than droxidopa but that midodrine but not droxidopa increased the risk of high blood pressure when lying down.[7] Small studies have also shown that midodrine can be used to prevent excessive drops in blood pressure in people requiring dialysis.[8]
Midodrine has been used in the complications of cirrhosis. It is also used with octreotide for hepatorenal syndrome; the proposed mechanism is constriction of splanchnic vessels and dilation of renal vasculature. Studies have not been sufficiently well conducted to show a clear place for midodrine.[9]
Contraindications
Midodrine is contraindicated in patients with severe organic heart disease, acute kidney disease, urinary retention, pheochromocytoma or thyrotoxicosis. Midodrine should not be used in patients with persistent and excessive supine hypertension.[10]
Side effects
Headache, feeling of pressure/fullness in the head, vasodilation/flushing face, scalp tingling, confusion/thinking abnormality, dry mouth, nervousness/anxiety and rash.[11]
Pharmacology
Mechanism of action
Midodrine is a prodrug which forms an active metabolite, desglymidodrine, which is an α1-receptor agonist and exerts its actions via activation of the alpha-adrenergic receptors of the arteriolar and venous vasculature, producing an increase in vascular tone and elevation of blood pressure. Desglymidodrine does not stimulate cardiac beta-adrenergic receptors. Desglymidodrine diffuses poorly across the blood–brain barrier, and is therefore not associated with effects on the central nervous system.
Pharmacokinetics
After oral administration, midodrine is rapidly absorbed. The plasma levels of the prodrug peak after about half an hour, and decline with a half-life of approximately 25 minutes, while the metabolite reaches peak blood concentrations about 1 to 2 hours after a dose of midodrine and has a half-life of about 3 to 4 hours. The absolute bioavailability of midodrine (measured as desglymidodrine) is 93%.[12]
Chemistry
Midodrine is an odorless, white, crystalline powder, soluble in water and sparingly soluble in methanol.
Stereochemistry
Midodrine contains a stereocenter and consists of two enantiomers, making it a racemate; i.e., a 1:1 mixture of (R)- and (S)-forms:[13]
| Enantiomers of midodrine | |
|---|---|
-Midodrin_Structural_Formula_V1.svg.png.webp) (R)-midodrine CAS number: 133163-25-4 |
-Midodrin_Structural_Formula_V1.svg.png.webp) (S)-midodrine CAS number: 133267-39-7 |
Synthesis
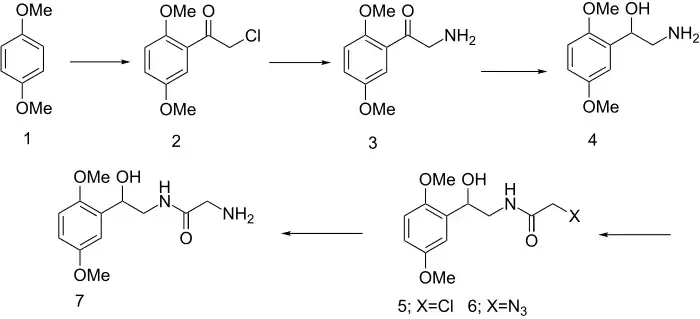
Acylation of 1,4-dimethoxybenzene with chloroacetyl chloride gives the chloroketone 2. The halogen is then converted to the amine 3 by any set of standard schemes, and the ketone reduced to an alcohol with borohydride (4). Acylation of the amino group in this last intermediate with chloroacetyl chloride affords the amide 5. The halogen is then displaced with azide and the resulting product 6 reduced catalytically to the glycinamide, midodrine (7).
References
- "Proamatine- midodrine hydrochloride tablet". DailyMed. Retrieved 14 August 2021.
- U.S. proposes withdrawal of Shire hypotension drug, 16 August 2010.
- O'Riordan, Michael. "FDA recommends withdrawal of midodrine". Food and Drug Administration. FDA proposes withdrawal of low blood pressure drug [press release]. August 16, 2010. TheHeart.org. Retrieved 1 April 2011.
- Midodrine (ProAmatine, generic) Proposed Market Withdrawal – Update 10 September 2010.
- plc, Shire. "Shire Provides Update on ProAmatine® (midodrine HCl)". www.prnewswire.com.
- Izcovich, A.; Gonzalez Malla, C.; Manzotti, M.; Catalano, H. N.; Guyatt, G. (22 August 2014). "Midodrine for orthostatic hypotension and recurrent reflex syncope: A systematic review". Neurology. 83 (13): 1170–1177. doi:10.1212/WNL.0000000000000815. PMID 25150287. S2CID 5439767.
- Chen JJ, Han Y, Tang J, Portillo I, Hauser RA, Dashtipour K (July 2018). "Standing and Supine Blood Pressure Outcomes Associated With Droxidopa and Midodrine in Patients With Neurogenic Orthostatic Hypotension: A Bayesian Meta-analysis and Mixed Treatment Comparison of Randomized Trials". Ann Pharmacother. 52 (12): 1182–1194. doi:10.1177/1060028018786954. PMID 29972032. S2CID 49674644.
- Prakash, S; Garg, AX; Heidenheim, AP; House, AA (October 2004). "Midodrine appears to be safe and effective for dialysis-induced hypotension: a systematic review". Nephrology, Dialysis, Transplantation. 19 (10): 2553–8. doi:10.1093/ndt/gfh420. PMID 15280522.
- Karwa, R.; Woodis, C B. (31 March 2009). "Midodrine and Octreotide in Treatment of Cirrhosis-Related Hemodynamic Complications". Annals of Pharmacotherapy. 43 (4): 692–699. doi:10.1345/aph.1L373. PMID 19299324. S2CID 207263346.
- "Midodrine - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 30 June 2022.
- "Midodrine (Oral Route) Side Effects - Mayo Clinic". www.mayoclinic.org.
- "Midodrine".
- Rote Liste Service GmbH (Hrsg.): Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). Rote Liste Service GmbH, Frankfurt/Main, 2017, Aufl. 57, ISBN 978-3-946057-10-9, S. 196.
- G. Zoelss, DE 2506110 (1976) via Chem. Abstr., 85:159,718s (1975).
- K. Wismayr et al., AT 241435; eidem, U.S. Patent 3,340,298 (1965, 1967 both to Chemie Linz Ag).
- Zoelss & W. Karl-Anton Ing DE 2523735 (1974 to Lentia GMBH).
External links
![]() Media related to Midodrine at Wikimedia Commons
Media related to Midodrine at Wikimedia Commons
- "Midodrine". Drug Information Portal. U.S. National Library of Medicine.