Amibegron
Amibegron (SR-58,611A) was a drug developed by Sanofi-Aventis (now Sanofi) which acts as a selective agonist for the β3 adrenergic receptor. It is the first orally active β3 agonist developed that is capable of entering the central nervous system, and has antidepressant and anxiolytic effects.[1][2]
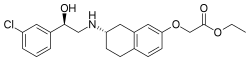 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H26ClNO4 |
| Molar mass | 403.90 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
On July 31, 2008, Sanofi-Aventis announced that it has decided to discontinue development of amibegron.[3]
References
- Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, et al. (February 2008). "Stimulation of the beta3-Adrenoceptor as a novel treatment strategy for anxiety and depressive disorders". Neuropsychopharmacology. 33 (3): 574–87. doi:10.1038/sj.npp.1301424. PMID 17460614.
- Overstreet DH, Stemmelin J, Griebel G (June 2008). "Confirmation of antidepressant potential of the selective beta3 adrenoceptor agonist amibegron in an animal model of depression". Pharmacology, Biochemistry, and Behavior. 89 (4): 623–6. doi:10.1016/j.pbb.2008.02.020. PMID 18358519. S2CID 35026036.
- Second quarter 2008 results. July 31, 2008, retrieved March 9, 2009.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.