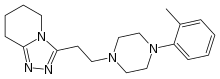
Dapiprazole
Dapiprazole (brand name Rev-Eyes) is an alpha-1 blocker. It is used to reverse mydriasis after eye examination.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601043 |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible when administered topically |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H27N5 |
| Molar mass | 325.460 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
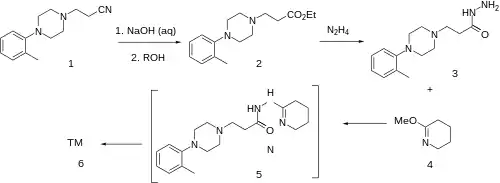
Synthesis
Dapiprazole uses a sidechain building strategy that was also used for Lorpiprazole
Conjugate addition of acrylonitrile to ortho-tolylpiperazine affords 3-[4-(2-methylphenyl)piperazin-1-yl]propanenitrile [65876-30-4] (1) in 99% yield. Hydrolysis of the nitrile group to an acid and subsequent esterification with ethanol gives ethyl 3-[4-(2-methylphenyl)piperazin-1-yl]propanoate, CID:417142 (2) .
Ex 2: Upon treatment with hydrazine, ester FGI to the hydrazide (3) CID:12785764 occurs. This is then reacted with the iminoether of 2-piperidone, i.e. o-methylvalerolactim [5693-62-9] (3). An unisolated intermediate such as (5), formed by an addition–elimination (curly arrows not displayed) is likely to account for the reaction mechanism sequence. The second step involves an attack on the carbonyl group by the now quite basic ring amidine nitrogen.
References
- Doughty MJ, Lyle WM (May 1992). "A review of the clinical pharmacokinetics of pilocarpine, moxisylyte (thymoxamine), and dapiprazole in the reversal of diagnostic pupillary dilation". Optometry and Vision Science. 69 (5): 358–68. doi:10.1097/00006324-199205000-00005. PMID 1350669. S2CID 22781280.
- DE2915318 idem Bruno Silvestrini, Leandro Baiocchi, U.S. Patent 4,252,721 & U.S. Patent 4,325,952 (1981 & 1982 both to Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.P.A.).