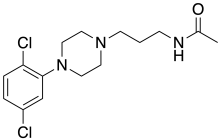
Acaprazine
Acaprazine (INN) is an anxiolytic and "adrenolytic" drug of the phenylpiperazine group that was never marketed.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H21Cl2N3O |
| Molar mass | 330.25 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Synthesis
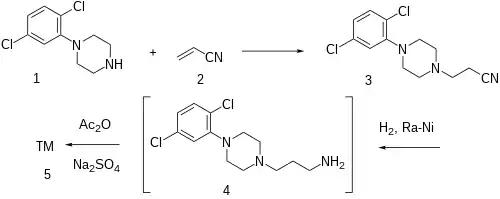
Patent:[2]
The Michael addition of acrylonitrile to 1-(2,5-Dichlorophenyl)Piperazine [1013-27-0] (1) gives the corresponding propionitrile (2).
Ex 1: Catalytic reduction over Raney nickel is performed in the presence of anhydrous sodium sulfate and acetic anhydride. This is a one-pot procedure for reducing the nitrile and mono acetylation of the primary amine produced in situ.
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 488. ISBN 978-1-4757-2085-3.
- Giuseppe Palazzo, Bruno Silvestrini, DE 2432030 (1975 to Acraf).
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.