Ranolazine
Ranolazine, sold under the brand name Ranexa among others, is a medication used to treat heart related chest pain.[2] Typically it is used together with other medications when those are insufficient.[2][3] Benefits appear smaller in women than men.[2] It is taken by mouth.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Ranexa, Aspruzyo Sprinkle, Corzyna |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606015 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35 to 50% |
| Protein binding | ~62% |
| Metabolism | Extensive in liver (CYP3A, CYP2D6) and intestine |
| Elimination half-life | 7 hours |
| Excretion | Kidney (75%) and fecal (25%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.259 |
| Chemical and physical data | |
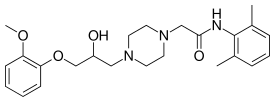
| Formula | C24H33N3O4 |
| Molar mass | 427.545 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Common side effects include constipation, headache, nausea, and dizziness.[2] Serious side effects may include QT prolongation.[2] Use is not recommended in those with liver cirrhosis.[2] How it works is not clear but may involve adenosine triphosphate.[2]
Ranolazine was approved for medical use in the United States in 2006.[2] In 2019, it was the 211th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[4][5]
Medical uses
Ranolazine is used to treat chronic angina.[6] It may be used concomitantly with β blockers, nitrates, calcium channel blockers, antiplatelet therapy, lipid-lowering therapy, ACE inhibitors, and angiotensin receptor blockers.[7] It is also effective at preventing atrial fibrillation, and has been studied as monotherapy as well as in combination with other medications used to treat irregular heartbeat.[8][9]
Its use is not recommended in Scotland as of 2019.[3]
Contraindications
Some contraindications for ranolazine are related to its metabolism and are described under Drug Interactions. Additionally, in clinical trials ranolazine slightly increased QT interval in some patients[10] and the FDA label contains a warning for doctors to beware of this effect in their patients.[7] The drug's effect on the QT interval is increased in the setting of liver dysfunction; thus it is contraindicated in persons with mild to severe liver disease.[11]
Side effects
The most common side effects are dizziness (11.5%) and constipation (10.9%).[6] Other side effects include headache and nausea.[10]
Drug interactions
Ranolazine is metabolized mainly by the CYP3A enzyme. It also inhibits another metabolizing enzyme, cytochrome CYP2D6.[7] For this reason, the doses of ranolazine and drugs that interact with those enzymes need to be adjusted when they are used by the same patient.
Ranolazine should not be used with drugs such as ketoconazole, clarithromycin, and nelfinavir that strongly inhibit CYP3A, nor with drugs that activate CYP3A, such as rifampin and phenobarbital.[7]
For drugs that are moderate CYP3A inhibitors, such as diltiazem, verapamil, and erythromycin, the dose of ranolazine should be reduced.[7]
Drugs that are metabolized by CYP2D6, such as tricyclic antidepressants, may need to be given at reduced doses when administered with ranolazine.[7]
Mechanism of action
Ranolazine inhibits persistent or late inward sodium current (INa) in heart muscle[12] in a variety of voltage-gated sodium channels.[13] Inhibiting that current leads to reductions in intracellular calcium levels. This in turn leads to reduced tension in the heart wall, leading to reduced oxygen requirements for the muscle.[10] The QT prolongation effect of ranolazine on the surface electrocardiogram is the result of inhibition of IKr, which prolongs the ventricular action potential.[7] Ranolazine also exhibits its effects on the delayed rectifier current (hERG/IKr potassium channels), it readily stimulates myogenesis, it reduces a pro-oxidant inflammation/oxidative condition, and activates the calcium signaling pathway.[14]
Ranolazine prolongs the action potential duration, with corresponding QT interval prolongation on electrocardiography, blocks the INa current, and prevents calcium overload caused by the hyperactive INa current, thus it stabilizes the membrane and reducing excitability.[15]
History
Syntex Inc. originally began developing ranolazine in 1985 and 61 studies were completed from then until 1994. Afterwards, Phase 2 studies were done however it was found that the formulation did not result in adequate plasma concentrations of drug. It is due to this that the sustained-release (SR) formulation of ranolazine was created.[16]
Roche acquired Syntex in 1994[16] In 1996, CV Therapeutics licensed the North American and European rights to ranolazine from Syntex, a subsidiary of Roche, which had discovered the drug and had developed it through Phase II trials in angina.[17] In 2006, CV Therapeutics acquired the remaining worldwide rights to ranolazine from Roche.[18] In 2008 CV Therapeutics exclusively licensed rights for ranolazine in Europe and some other countries to Menarini.[19] In 2009, Gilead acquired CV Therapeutics.[20] In 2013 Gilead expanded the partnership with Menarini to include additional countries, including those in Asia.[21]
Society and culture
Legal status
Ranolazine was approved by the FDA in January 2006, for the treatment of patients with chronic angina as a second-line treatment in addition to other drugs.[10] In 2007 the label was updated to make ranolazine a first-line treatment, alone or with other drugs.[10] In April 2008 ranolazine was approved by the European EMEA for use in angina.[22]
Commercial aspects
Ranolazine is manufactured and sold as Ranexa by Gilead. According to a Gilead annual income statement, combined sales for Ranexa and another Gilead product, AmBisom, were $621 million for the fourth quarter of 2016.[23]
References
- "Summary Basis of Decision (SBD) for Corzyna". Health Canada. Retrieved 29 May 2022.
- "Ranolazine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 22 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 210. ISBN 9780857113382.
- "The Top 300 of 2019". ClinCalc. Retrieved 16 October 2021.
- "Ranolazine - Drug Usage Statistics". ClinCalc. Retrieved 16 October 2021.
- Banon D, Filion KB, Budlovsky T, Franck C, Eisenberg MJ (March 2014). "The usefulness of ranolazine for the treatment of refractory chronic stable angina pectoris as determined from a systematic review of randomized controlled trials". The American Journal of Cardiology. 113 (6): 1075–82. doi:10.1016/j.amjcard.2013.11.070. PMID 24462341.
- "Ranexa (ranolazine) Extended-Release Tablets, for Oral Use. Full Prescribing Information". Gilead Sciences, Inc. Foster City, CA 94404. Retrieved 8 September 2016.
- Rosa, Gian Marco; Dorighi, Ulrico; Ferrero, Simone; Brunacci, Michele; Bertero, Giovanni; Brunelli, Claudio (June 2015). "Ranolazine for the treatment of atrial fibrillation". Expert Opinion on Investigational Drugs. 24 (6): 825–836. doi:10.1517/13543784.2015.1036984. ISSN 1744-7658. PMID 25872749. S2CID 31659714.
- De Vecchis, Renato; Ariano, Carmelina; Giasi, Anna; Cioppa, Carmela (June 2018). "Antiarrhythmic effects of ranolazine used both alone for prevention of atrial fibrillation and as an add-on to intravenous amiodarone for its pharmacological cardioversion: a meta-analysis". Minerva Cardioangiologica. 66 (3): 349–359. doi:10.23736/S0026-4725.17.04349-3. ISSN 1827-1618. PMID 28497941.
- Kloner RA, Hines ME, Geunes-Boyer S (November 2013). "Efficacy and safety of ranolazine in patients with chronic stable angina". Postgraduate Medicine. 125 (6): 43–52. doi:10.3810/pgm.2013.11.2711. PMID 24200760. S2CID 2084618.
- "FDA Approves New Treatment for Chest Pain". FDA News. 31 January 2006. Retrieved 2 March 2011.
- Noble D, Noble PJ (July 2006). "Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload". Heart. 92 Suppl 4: iv1–iv5. doi:10.1136/hrt.2005.078782. PMC 1861316. PMID 16775091.
- Sokolov S, Peters CH, Rajamani S, Ruben PC (2013). "Proton-dependent inhibition of the cardiac sodium channel Nav1.5 by ranolazine". Frontiers in Pharmacology. 4: 78. doi:10.3389/fphar.2013.00078. PMC 3689222. PMID 23801963.
- Thomsen MB, Matz J, Volders PG, Vos MA (October 2006). "Assessing the proarrhythmic potential of drugs: current status of models and surrogate parameters of torsades de pointes arrhythmias". Pharmacology & Therapeutics. 112 (1): 150–70. doi:10.1016/j.pharmthera.2005.04.009. PMID 16714061.
- Banerjee K, Ghosh RK, Kamatam S, Banerjee A, Gupta A (January 2017). "Role of Ranolazine in cardiovascular disease and diabetes: Exploring beyond angina". International Journal of Cardiology. 227: 556–564. doi:10.1016/j.ijcard.2016.10.102. PMID 27838121.
- "Cardiovascular and Renal Drugs Advisory Committee Briefing Document" (PDF). Food and Drug Administration.
- CV Therapeutics press release. April 1, 1996 CV Therapeutics Licenses Late-Stage Anti-Anginal Drug from Syntex (U.S.A.), an Affiliate of Roche Holding Ltd.
- CV Therapeutics, 22 June 2006 CV Therapeutics Acquires Rights to Ranolazine in Asia
- Thepharmaletter.com 22 September 2008 Italy's Menarini to pay up to $385 million for rights to CV Thera's Ranexa
- Reuters, via the New York Times. 12 March 2009. Gilead, a White Knight, to Buy CV Therapeutics
- Menarini press release. 18 June 2013 Memarii Group announces agreement with Gilead Sciences to commercialize Ranexa® (ranolazine) in 50 new countries
- EMEA Ranolazine page at the EMEA
- "Gilead Sciences Announces Fourth Quarter and Full Year 2016 Financial Results". Gilead.
External links
- "Ranolazine". Drug Information Portal. U.S. National Library of Medicine.