Benzamil
Benzamil or benzyl amiloride is a potent blocker of the ENaC channel[1] and also a sodium-calcium exchange blocker.[2][3] It is a potent analog of amiloride, and is marketed as the hydrochloride salt (benzamil hydrochloride). As amiloride, benzamil has been studied as a possible treatment for cystic fibrosis,[4] although with disappointing results.[5]
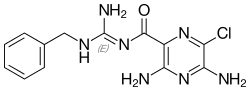 | |
| Names | |
|---|---|
| IUPAC name
3,5-diamino-N-[(1E)-amino(benzylamino)methylidene]-6-chloropyrazine-2-carboxamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | benzamil |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C13H14ClN7O |
| Molar mass | 319.75 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
Benzamil is a benzyl group-containing analog of amiloride. Like amiloride, it is a guanidinium group-containing pyrazine derivative.
Mechanism of action
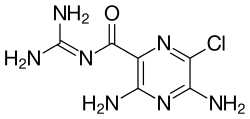
Benzamil is closely related to amiloride. By adding the benzyl group to the nitrogen of the guanidinium group the activity is increased several hundredfold.[6]
Amiloride works by directly blocking the epithelial sodium channel (ENaC) thereby inhibiting sodium reabsorption in the distal convoluted tubules and collecting ducts in the kidneys (this mechanism is the same for triamterene). This promotes the loss of sodium and water from the body, but without depleting potassium.
References
- Chalfant, M.L. (1995). "Regulation of epithelial Na+ channels from M-1 cortical collecting duct cells". American Journal of Physiology. Renal Physiology. 271 (4): f861–f870. doi:10.1152/ajprenal.1996.271.4.f861. PMID 8898016.
- Gomez-Sanchez, E. P.; Gomez-Sanchez C. E. (September 1995). "Effect of central infusion of benzamil on Dahl S rat hypertension". Am J Physiol. 269 (3, pt 2): H1044–7. doi:10.1152/ajpheart.1995.269.3.H1044. PMID 7573500.
- Lee, Y. S.; Sayeed, M. M.; Wurster, R. D. (January 6, 1995). "Intracellular Ca2+ mediates the cytotoxicity induced by bepridil and benzamil in human brain tumor cells". Cancer Letters. 88 (1): 87–91. doi:10.1016/0304-3835(94)03619-T. PMID 7850778. Archived from the original on August 7, 2008. Retrieved 2008-05-01.
- Rodgers HC, Knox AJ (September 1999). "The effect of topical benzamil and amiloride on nasal potential difference in cystic fibrosis". Eur. Respir. J. 14 (3): 693–6. doi:10.1034/j.1399-3003.1999.14c32.x. PMID 10543294.
- Hirsh AJ, Sabater JR, Zamurs A, et al. (December 2004). "Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease". J. Pharmacol. Exp. Ther. 311 (3): 929–38. doi:10.1124/jpet.104.071886. PMID 15273255. S2CID 3160146.
- Kleyman, T. R.; Cragoe E. J. Jr. (October 1988). "Amiloride and its analogs as tools in the study of ion transport". J Membr Biol. 105 (1): 1–21. doi:10.1007/BF01871102. PMID 2852254. S2CID 21071525.