Phenacemide
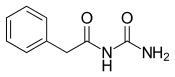
Phenacemide (INN, BAN) (brand name Phenurone), also known as phenylacetylurea, is an anticonvulsant of the ureide (acetylurea) class.[1] It is a congener and ring-opened analogue of phenytoin (a hydantoin),[2][3] and is structurally related to the barbiturates and to other hydantoins.[4] Phenacemide was introduced in 1949 for the treatment of epilepsy, but was eventually withdrawn due to toxicity.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Phenurone |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 22–25 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.519 |
| Chemical and physical data | |
| Formula | C9H10N2O2 |
| Molar mass | 178.191 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1578–. ISBN 978-0-412-46630-4.
- Conceptual Pharmacology. Universities Press. 2010. pp. 236–. ISBN 978-81-7371-679-9.
- deStevens, G.; Zingel, V.; Leschke, C.; Hoeprich, P.D.; Schultz, R.M.; Mehrotra, P.K.; Batra, S.; Bhaduri, A.P.; Saxena, A.K.; Saxena, M., eds. (11 November 2013). Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des Recherches Pharmaceutiques. Basel: Birkhäuser. pp. 217–. ISBN 978-3-0348-7161-7. Retrieved 3 September 2016.
- S. S. Kadam (1 July 2007). PRINCIPLES OF MEDICINAL CHEMISTRY Vol. - II. Pragati Books Pvt. Ltd. pp. 147–. ISBN 978-81-85790-03-9.
External links
- Diseases Database (DDB): 34078
- MedlinePlus DrugInfo uspdi-202454
- Coker S (1986). "The use of phenacemide for intractable partial complex epilepsy in children". Pediatr Neurol. 2 (4): 230–2. doi:10.1016/0887-8994(86)90053-6. PMID 3508693.
- Coker S, Holmes E, Egel R (1987). "Phenacemide therapy of complex partial epilepsy in children: determination of plasma drug concentrations". Neurology. 37 (12): 1861–6. doi:10.1212/wnl.37.12.1861. PMID 3683877. S2CID 219205208.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.