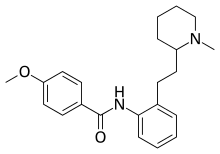
Encainide
Encainide (trade name Enkaid) is a class Ic antiarrhythmic agent. It is no longer used because of its frequent proarrhythmic side effects.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Enkaid |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a605040 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H28N2O2 |
| Molar mass | 352.478 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
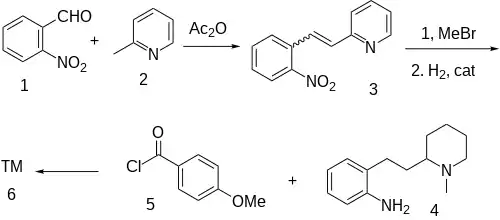
The condensation of 2-picoline [109-06-8] (1) with 2-nitrobenzaldehyde [552-89-6] (2) catalyzed with acetic anhydride gives 2-[2-(2-nitrophenyl)ethenyl]pyridine, CID:142706 {aka 2-(o-nitrostyryl)pyridine} [77340-84-2] (3). N-Methylation followed by the reduction the aromaticity in the pyridine ring as well as the nitro group to the corresponding aniline gives 2-(2-aminophenethyl)-1-methylpiperidine aka 2-[2-(1-methylpiperidin-2-yl)ethyl]aniline, [37611-80-6] CID:11085282 (4). Amide formation with p-anisoyl chloride [100-07-2] (5) completed the synthesis of Encainide (6).
See also
- Iferanserin
- Modecainide [81329-71-7]
- Cardiac Arrhythmia Suppression Trial
References
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. (March 1991). "Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial". The New England Journal of Medicine. 324 (12): 781–8. doi:10.1056/NEJM199103213241201. PMID 1900101.
- Dykstra SJ, Minielli JL, Lawson JE, Ferguson HC, Dungan KW (September 1973). "Lysergic acid and quinidine analogs. 2-(o-Acylaminophenethyl)piperidines". Journal of Medicinal Chemistry. 16 (9): 1015–20. doi:10.1021/jm00267a012. PMID 4745503.
- DE 2210154, Dykstra SJ, Minelli JL, issued 1972
- US 3931195, Dykstra SJ, Minelli JL, issued 1976
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.