Lidoflazine
Lidoflazine is a piperazine calcium channel blocker. It is a coronary vasodilator with some antiarrhythmic action.[1] Lidoflazine was discovered at Janssen Pharmaceutica in 1964.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.285 |
| Chemical and physical data | |
| Formula | C30H35F2N3O |
| Molar mass | 491.627 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 159 to 161 °C (318 to 322 °F) |
| Solubility in water | Almost insoluble in water(<0.01%); Very soluble in chloroform(>50%); mg/mL (20 °C) |
SMILES
| |
InChI
| |
| | |
Physical properties
Solubility at room temperature
Extracted from[1]
| Solvent | 0.01
N |
0.1
N | ||
| % | pH | % | pH | |
| Hydrochloric Acid | 0.4 | 3.0 | 0.7 | 1.9 |
| Tartaric Acid | 0.3 | 3.1 | 1.0 | 2.5 |
| Citric Acid | 0.3 | 3.1 | 0.5 | 2.5 |
| Lactic Acid | 0.2 | 3.4 | 0.7 | 2.9 |
| Acetic Acid | 0.1 | 3.5 | 0.4 | 3.8 |
Synthesis
Compare for Ranolazine.
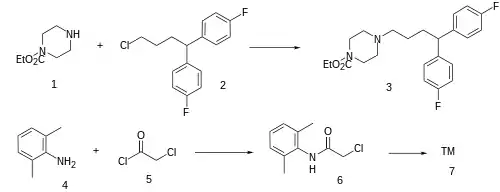
Alkylation of N-carbethoxypiperazine [120-43-4] (1) with 1-[4-chloro-1-(4-fluorophenyl)butyl]-4-fluorobenzene [3312-04-7] (2) gives CID:12679861 (3). Deprotection of which afforded 1-[4,4-bis(4-fluorophenyl)butyl]piperazine [5631-35-6]. Acylation of 2,6-xylidine (4) with chloroacetyl chloride (5) gives N-chloroacetyl-2,6-xylidine [1131-01-7] (6). The convergent synthesis between the above two counterparts completed the synthesis of lidoflazine (7).
References
- Schaper WK, Xhoneux R, Jageneau AH, Janssen PA (May 1966). "The cardiovascular pharmacology of lidoflazine, a long-acting coronary vasodilator". The Journal of Pharmacology and Experimental Therapeutics. 152 (2): 265–74. PMID 5944369.
- NL6507312 idem Hermans Hubert Karel Frans, Schaper Wolfgang Karl-Adolf, U.S. Patent 3,267,104 (1966 to Janssen Pharmaceutica Nv).
- Anders K. Bjork & Erik G. Christensson, WO 1983001950 (to Pharmacia and Upjohn Animal Health AB).
Further reading
- Schaper WK, Xhonneux R, Jageneau AH (November 1965). "Stimulation of the coronary collateral circulation by lidoflazine (R 7904)". Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie. 252 (1): 1–8. doi:10.1007/bf00246424. PMID 4222721. S2CID 31959581.
- Zhou, Ping-Zheng; Babcock, Joseph; Liu, Lian-Qing; Li, Min; Gao, Zhao-Bing (2011). "Activation of human ether-a-go-go related gene (HERG) potassium channels by small molecules". Acta Pharmacologica Sinica. 32 (6): 781–788. doi:10.1038/aps.2011.70. PMC 4085723. PMID 21623390.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.