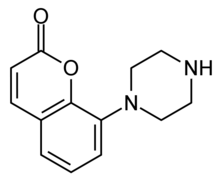
Batoprazine
Batoprazine is a drug of the phenylpiperazine class which has been described as a serenic or antiaggressive agent.[1][2] It acts as a 5-HT1A and 5-HT1B receptor agonist.[2][3] It is closely related to eltoprazine, fluprazine, and naphthylpiperazine, of which possess similar actions and effects.[3]
 | |
| Clinical data | |
|---|---|
| Other names | 8-(1-piperazinyl)coumarin; 8-(1-piperazinyl)-2H- |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H14N2O2 |
| Molar mass | 230.267 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
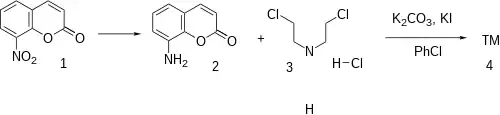
Synthesis
- 8-Nitrocoumarin can be obtained in various methods, for example through nitration of coumarin.
- 8-Aminocoumarin was obtained from reduction of the corresponding nitrated derivative.
- Ex 7 to 10: The 8-aminocoumarin [75487-97-7] (1) is reacted with an excess of bis(2-chloroethyl)amine hydrochloride [821-48-7] (2) in the presence of potassium carbonate and then of potassium iodide, the reaction being carried out under reflux in chlorobenzene.
See also
- Phenylpiperazine
References
- Olivier B (December 2004). "Serotonin and aggression". Annals of the New York Academy of Sciences. 1036: 382–92. doi:10.1196/annals.1330.022. PMID 15817750. S2CID 45595253.
- Olivier B, van Oorschot R (December 2005). "5-HT1B receptors and aggression: a review". European Journal of Pharmacology. 526 (1–3): 207–17. doi:10.1016/j.ejphar.2005.09.066. PMID 16310769.
- Gommans J, Hijzen TH, Maes RA, Olivier B (1997). "Discriminative stimulus properties of eltoprazine". Life Sciences. 61 (1): 11–9. doi:10.1016/S0024-3205(97)00352-4. PMID 9200664.
- Jean-Louis Peglion, Mark Millan, Jean-Michel Rivet, U.S. Patent 5,194,437 (1993 to Adir Et Compagnie).
- Reppel, L.; Schmollack, W. (1963). "Beiträge zur Kenntnis der Cumarine. 2. Mitt.: Die Darstellung von Aminocumarinen". Archiv der Pharmazie. 296 (6): 365–369. doi:10.1002/ardp.19632960603.
- Shockravi, A., Valizadeh, H., Heravi, M. M. (1 March 2003). "A One-Pot and Convenient Synthesis of Coumarins in Solventless System". Phosphorus, Sulfur, and Silicon and the Related Elements. 178 (3): 501–504. doi:10.1080/10426500307915.
- Ramana, M. M. V., Malik, S. S., Parihar, J. A. (November 2004). "Guanidinium nitrate: a novel reagent for aryl nitrations". Tetrahedron Letters. 45 (47): 8681–8683. doi:10.1016/j.tetlet.2004.09.140.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.