AP-238
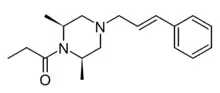
AP-238 is an opioid designer drug related to drugs such as azaprocin and bucinnazine, with around the same potency as morphine.[1] It was first discovered in Italy in the 1960s but was never marketed,[2] subsequently appearing on the illicit market around 2020 and being detected in both Slovenia and the USA.[3][4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H26N2O |
| Molar mass | 286.419 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Fogarty, Melissa F.; Vandeputte, Marthe M.; Krotulski, Alex J.; Papsun, Donna; Walton, Sara E.; Stove, Christophe P.; Logan, Barry K. (2022). "Toxicological and pharmacological characterization of novel cinnamylpiperazine synthetic opioids in humans and in vitro including 2-methyl AP-237 and AP-238". Archives of Toxicology. doi:10.1007/s00204-022-03257-7. ISSN 1432-0738. PMID 35275255.
- Cignarella G, Testa E (May 1968). "2,6-Dialkylpiperazines. IV. 1-Propionyl-4-substituted cis-2,6-dimethylpiperazines structurally related to the analgetic 8-acyl-3,8-diazabicyclo[3.2.1]octanes". Journal of Medicinal Chemistry. 11 (3): 592–4. doi:10.1021/jm00309a039. PMID 5656502.
- "Analytical Report. AP-238. 1‐{2,6‐dimethyl‐4‐[(2E)‐3‐phenylprop‐2‐en‐1‐yl]piperazin‐1‐yl}propan‐1‐one" (PDF). Nacionalni Forenzični Laboratorij. 20 October 2020.
- "AP-238" (PDF). NPS Discovery at CFSRE. Center for Forensic Science Research and Education (CFSRE). 11 November 2020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.