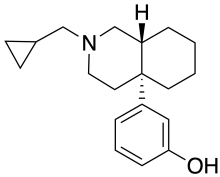
Ciprefadol
Ciprefadol is an opioid analgesic that is an isoquinoline derivative most closely related to cyclazocine and picenadol,[1] with a number of other related compounds known.[2][3][4][5] Ciprefadol is a mixed agonist–antagonist at μ-opioid receptors and can partly block the effects of morphine at low doses, though at higher doses it acts more like a full agonist. It is also a potent κ-opioid agonist, unlike the corresponding N-methyl and N-phenethyl derivatives which are reasonably μ-selective agonists.[6]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H27NO |
| Molar mass | 285.431 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
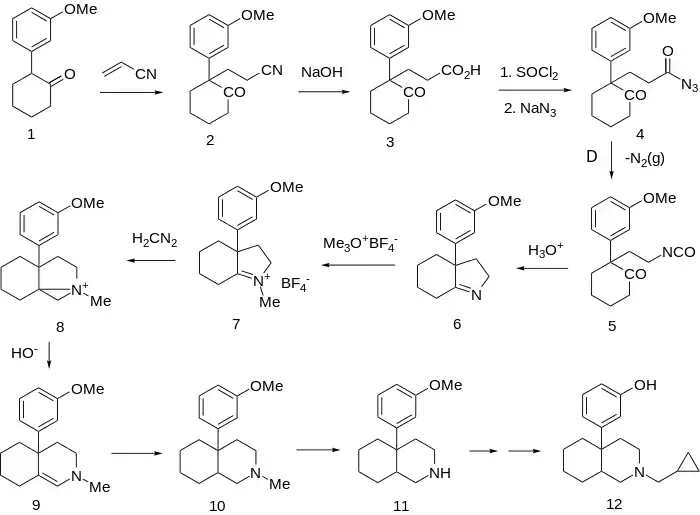
Fusion of an alicyclic ring onto the piperidine so as to form a perhydroisoquinoline is apparently consistent with analgesic activity.
The Michael addition between 2-(3-Methoxyphenyl)Cyclohexanone [15547-89-4] (1) and acrylonitrile gives CID:20460008 (2). Hydrolysis of the nitrile to the corresponding acid gives CID:20334481 (3). FGI to the acyl azide (4) followed by Curtius rearrangement leads to isocyanate CID:85857788 (5). Acid hydrolysis leads directly to the indoline, CID:427266 (6), by internal Schiff base formation of the intermediate amine.
Methylation by means of trimethyloxonium tetrafluoroborate affords ternary iminium salt (7). Treatment of that reactive carbonyl-like functionality with diazomethane gives the so-called azonia salt (8) (note the analogy to the hypothetical oxirane involved in ring expansion of ketones with diazomethane).
Exposure of the aziridinium intermediate to base leads to ring opening and consequent formation of the octahydroisoquinoline, CID:12884444 (9). Reduction of the enamine (catalytic or borohydride) affords the perhydroisoquinoline [58414-81-6] (10). The N-demethylation sequence gives CID:13640225 (11). O-Demethylation of the phenol ether gives CID:14020576.
Alkylation with cyclopropylcarbonylchloride [4023-34-1] followed by reduction of the amide with lithium borohydride completes the preparation of ciprefadol (12).
References
- Dennis M. Zimmerman et al. N-cycloalkylmethyl decahydroisoquinolines. US Patent 4001248, Jun 7, 1974
- David R. Brittelli et al. 4a-Aryl-trans-decahydroisoquinolines. US Patent 4419517, Apr 8, 1975
- Henry Rapoport et al. Synthesis of 4A-aryl-decahydroisoquinolines. US Patent 4189583, Apr 26, 1978
- Judd DB, Brown DS, Lloyd JE, McElroy AB, Scopes DI, Birch PJ, et al. (January 1992). "Synthesis, antinociceptive activity, and opioid receptor profiles of substituted trans-3-(decahydro- and octahydro-4a-isoquinolinyl)phenols". Journal of Medicinal Chemistry. 35 (1): 48–56. doi:10.1021/jm00079a005. PMID 1310115.
- Carroll FI, Chaudhari S, Thomas JB, Mascarella SW, Gigstad KM, Deschamps J, Navarro HA (December 2005). "N-substituted cis-4a-(3-hydroxyphenyl)-8a-methyloctahydroisoquinolines are opioid receptor pure antagonists". Journal of Medicinal Chemistry. 48 (26): 8182–93. doi:10.1021/jm058261c. PMC 2585695. PMID 16366600.
- Zimmerman DM, Cantrell BE, Swartzendruber JK, Jones ND, Mendelsohn LG, Leander JD, Nickander RC (March 1988). "Synthesis and analgesic properties of N-substituted trans-4a-aryldecahydroisoquinolines". Journal of Medicinal Chemistry. 31 (3): 555–60. doi:10.1021/jm00398a011. PMID 2831363.
- D. M. Zimmerman and VI. S. Marshall, U.S. Patent 4,029,796 (1977 to Eli Lilly).
- David R. Brittelli & William C. Ripka, U.S. Patent 4,419,517 (1983 to Bristol Myers Squibb Pharma Co).