Deltorphin
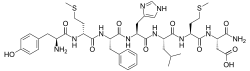
Deltorphin, also known as deltorphin A and dermenkephalin, is a naturally occurring, exogenous opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2.[1][2][3] Along with the other deltorphins (such as deltorphin I and deltorphin II) and the dermorphins, deltorphin is endogenous to frogs of the genus Phyllomedusa such as P. bicolor and P. sauvagei where it is produced in their skin, and is not known to occur naturally in any other species.[1][2][4] Deltorphin is one of the highest affinity and most selective naturally occurring opioid peptides known, acting as a very potent and highly specific agonist of the δ-opioid receptor.[1][2][3]
 | |
| Names | |
|---|---|
| IUPAC names
(3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]-4-(methylsulfanyl)butanamido]-3-phenylpropanamido]-3-(1H-imidazol-4-yl)propanamido]-4-methylpentanamido]-4-(methylsulfanyl)butanamido]-3-carbamoylpropanoic acid or L-tyrosyl-D-methionyl-L-phenylalanyl-L-histidyl-L-leucyl-L-methionyl-L-α-asparagine | |
| Other names
Deltorphin A; Dermenkephalin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C44H62N10O10S2 |
| Molar mass | 955.154 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Deltorphins have an unusually high blood–brain barrier penetration rate. The nonselective opiate antagonist naloxone inhibits deltorphin uptake by brain microvessels, but neither the selective δ-opioid antagonist naltrindole nor a number of opioid peptides with different affinities for δ- or μ-opioid receptors compete with deltorphins for the transport.[5]
See also
- Deltorphin I
- Dermorphin
References
- Kreil G, Barra D, Simmaco M, et al. (March 1989). "Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for delta opioid receptors". European Journal of Pharmacology. 162 (1): 123–8. doi:10.1016/0014-2999(89)90611-0. PMID 2542051.
- Mor A, Delfour A, Sagan S, et al. (September 1989). "Isolation of dermenkephalin from amphibian skin, a high-affinity delta-selective opioid heptapeptide containing a D-amino acid residue". FEBS Letters. 255 (2): 269–74. doi:10.1016/0014-5793(89)81104-4. PMID 2551734. S2CID 6095995.
- Erspamer V, Melchiorri P, Falconieri-Erspamer G, et al. (July 1989). "Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites". Proceedings of the National Academy of Sciences of the United States of America. 86 (13): 5188–92. Bibcode:1989PNAS...86.5188E. doi:10.1073/pnas.86.13.5188. PMC 297583. PMID 2544892.
- Temussi PA, Picone D, Tancredi T, et al. (April 1989). "Conformational properties of deltorphin: new features of the delta-opioid receptor". FEBS Letters. 247 (2): 283–8. doi:10.1016/0014-5793(89)81353-5. PMID 2541018. S2CID 84259225.
- Fiori, Anna; Cardelli, Patrizia; Negri, Lucia; Savi, Maria Rosaria; Strom, Roberto; Erspamer, Vittorio (1997-08-19). "Deltorphin transport across the blood–brain barrier". Proceedings of the National Academy of Sciences of the United States of America. 94 (17): 9469–9474. Bibcode:1997PNAS...94.9469F. doi:10.1073/pnas.94.17.9469. ISSN 0027-8424. PMC 23226. PMID 9256506.