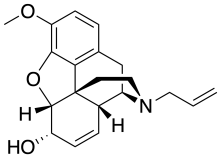
Nalodeine
Nalodeine, also known more commonly as N-allylnorcodeine, is an opioid antagonist (specifically, an antagonist of the μ-opioid receptor) that was never marketed but is notable as the first opioid antagonist to be discovered.[1][2] It was first reported in 1915 and was found to block the effects of morphine in animals.[3][2] This was followed by the clinical introduction of nalorphine (N-allylnormorphine) in 1954, naloxone (N-allyloxymorphone) in 1960, and naltrexone (N-methylcyclopropyloxymorphone) in 1963.[2] Nalmefene (6-desoxy-6-methylene-naltrexone), another structurally related opioid antagonist derivative, was also subsequently introduced, in 1996.[4] In animals, nalodeine both reverses morphine- and heroin-induced respiratory depression and acts as a respiratory stimulant in its own right (i.e., when given alone).[5] Similarly to nalorphine, nalodeine has also been found to act as an agonist of the κ-opioid receptor.[6]
 | |
| Clinical data | |
|---|---|
| Other names | N-Allylnorcodeine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H23NO3 |
| Molar mass | 325.408 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Gavril Pasternak (17 April 2013). The Opiate Receptors. Springer Science & Business Media. pp. 4–. ISBN 978-1-60761-990-1.
- Anil Aggrawal. APC Essentials of Forensic Medicine and Toxicology. Avichal Publishing Company. pp. 554–. ISBN 978-81-7739-441-2.
- Gonzalez JP, Brogden RN (March 1988). "Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence". Drugs. 35 (3): 192–213. doi:10.2165/00003495-198835030-00002. PMID 2836152.
- Robert M. Julien; Claire D. Advokat; Joseph E. Comaty (8 October 2010). Primer of Drug Action. Worth Publishers. pp. 349–. ISBN 978-1-4292-3343-9.
- Bentham Science Publishers (April 1995). Current Medicinal Chemistry. Bentham Science Publishers. pp. 423–.
- Cho N, Hirobe M, Takayanagi I (1985). "Effects of epoxidation on the actions of normorphine, norcodeine, N-allylnormorphine(nalorphine) and N-allylnorcodeine on the electrically stimulated guinea pig ileum" (PDF). Chem. Pharm. Bull. 33 (4): 1681–6. doi:10.1248/cpb.33.1681. PMID 4042244.