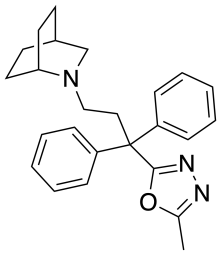
Nufenoxole
Nufenoxole (SC-27166) is an antidiarrhoeal drug which acts as a peripherally selective opioid agonist, in a similar manner to loperamide and diphenoxylate. While it is able to activate μ-opioid receptors, it fails to cross the blood-brain barrier and so has a selective action against diarrhoea without producing analgesic effects.[1][2][3][4][5]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H29N3O |
| Molar mass | 387.527 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- Dipipanone
- Dipyanone
- Desmethylmoramide
References
- US RE29556E, Adelstein GW, "1,1-Diaryl-1-oxadiazol-alkylamines", issued 28 February 1978, assigned to G.D. Searle & Company
- Dajani EZ, Bianchi RG, East PF, Bloss JL, Adelstein GW, Yen CH (December 1977). "The pharmacology of SC-27166: a novel antidiarrheal agent". The Journal of Pharmacology and Experimental Therapeutics. 203 (3): 512–26. PMID 411912.
- Mackerer CR, Brougham LR, East PF, Bloss JL, Dajani EZ, Clay GA (December 1977). "Antidiarrheal and central nervous system activities of SC-27166 (2-[3 - 5 - methyl - 1, 3, 4 - oxadiazol - 2 - yl) - 3, 3 - diphenylpropyl] - 2 - azabicyclo [2.2.2]octane), a new antidiarrheal agent, resulting from binding to opiate receptor sites of brain and myenteric plexus". The Journal of Pharmacology and Experimental Therapeutics. 203 (3): 527–38. PMID 200732.
- Niemegeers CJ, McGuire JL, Heykants JJ, Janssen PA (September 1979). "Dissociation between opiate-like and antidiarrheal activities of antidiarrheal drugs". The Journal of Pharmacology and Experimental Therapeutics. 210 (3): 327–33. PMID 480185.
- Korey A, Zilm DH, Sellers EM (May 1980). "Dependence liability of two antidiarrheals, nufenoxole and loperamide". Clinical Pharmacology and Therapeutics. 27 (5): 659–64. doi:10.1038/clpt.1980.93. PMID 7371363. S2CID 35222789.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.