Semax
Semax is a drug which is used mostly in Russia and Ukraine for a broad range of conditions but predominantly for its purported nootropic, neuroprotective, and neurorestorative properties. Semax has not been evaluated, approved for use, or marketed in most other countries.
 | |
| Clinical data | |
|---|---|
| Trade names | Semax |
| Other names | L-Methionyl-L-α-glutamylhistidyl-L-phenylalanyl-L-prolylglycyl-L-proline, (Pro8,Gly9,Pro10)ACTH-(4-10) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
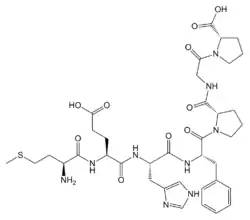
| Formula | C37H51N9O10S |
| Molar mass | 813.93 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Medical uses

Semax has undergone extensive study in Russia and is on the Russian List of Vital & Essential Drugs approved by the Russian Federation government on December 7, 2011.[1] Medical uses for Semax include treatment of stroke, transient ischemic attack, memory and cognitive disorders, peptic ulcers, optic nerve disease, and to boost the immune system.[2][3][4][5]
Pharmacology
Pharmacodynamics
In animals, Semax rapidly elevates the levels and expression of brain-derived neurotrophic factor (BDNF) and its signaling receptor TrkB in the hippocampus,[6] and rapidly activates serotonergic and dopaminergic brain systems.[7][8] Accordingly, it has been found to produce antidepressant-like and anxiolytic-like effects,[9][10] attenuate the behavioral effects of exposure to chronic stress,[9][10] and potentiate the locomotor activity produced by D-amphetamine.[8][11] As such, it has been suggested that Semax may be effective in the treatment of depression.[12]
Though the exact mechanism of action of Semax is unclear, there is evidence that it may act through melanocortin receptors. Specifically, there is a report of Semax competitively antagonizing the action of the melanocortin receptor full agonist α-melanocyte-stimulating hormone (α-MSH) at the MC4 and MC5 receptors in both in vitro and in vivo experimental conditions, indicating that it may act as an antagonist or partial agonist of these receptors.[13] Semax did not antagonize α-MSH at the MC3 receptor, though this receptor could still be a target of the drug.[13] As for the MC1 and MC2 receptors, they were not assayed.[13] In addition to actions at receptors, Semax, as well as a related peptide drug, Selank, have been found to inhibit enzymes involved in the degradation of enkephalins and other endogenous regulatory peptides (IC50 = 10 μM), though the clinical significance of this property is uncertain.[14]
Pharmacokinetics
As a peptide, Semax has poor oral bioavailability and hence is administered parenterally as a nasal spray or subcutaneous injection.
Human trials
In a 2018 study involving healthy participants, Semax was shown to increase fMRI default mode network activity relative to placebo.[15]
In an earlier 1996 study, 250-1000 ug/kg of Semax improved attention and short term memory in healthy subjects performing 8 hour work shifts, though the effects were most pronounced when subjects were fatigued (after their shift was over) and the effects lasted going into the next day.[16] In the memory test administered the morning after Semax administration, the treatment group made more correct responses (71%) than the control group (41%).[16]
Both studies had small sample sizes of 24 and 11 participants respectively.[15][16]
A study involving 110 patients recovering from ischemic stroke reported increases in BDNF (correlated with early rehabilitation) in patients administered Semax.[17]
As of September 2022, there are no published human trials involving Semax outside of Russia and Post-Soviet states.
Chemistry
Semax is a heptapeptide and synthetic analogue of a fragment of adrenocorticotropic hormone (ACTH), ACTH (4-10), of the following amino acid sequence: Met-Glu-His-Phe-Pro-Gly-Pro (MEHFPGP in single-letter form).
References
- "ПЕРЕЧЕНЬ. жизненно необходимых и важнейших лекарственных препаратов на 2012 год. (Vital and Essential Drugs List, 2012) - Russian Federation". World Health Organization. 2012. Archived from the original on February 2, 2015.
- "Semax". Institute of Molecular Genetics, Russian Academy of Sciences.
- Kurysheva NI; Shpak AA; Ioĭleva EE; Galanter LI; Nagornova ND; Shubina NIu; Shlyshalova NN (2001). "Semax in the treatment of glaucomatous optic neuropathy in patients with normalized ophthalmic tone". Vestnik Oftalmologii. 117 (4): 5–8. PMID 11569188.
- Ivanikov IO, Brekhova ME, Samonina GE, Myasoedov NF, Ashmarin IP (2002). "Therapy of peptic ulcer with semax peptide". Bulletin of Experimental Biology and Medicine. 134 (1): 73–4. doi:10.1023/A:1020621124776. PMID 12459874. S2CID 23447014.
- Korneev, EA; Kazakova, TB (1999). "Current approaches to the analysis of the effects of stress on metabolic processes in cells of the nervous and immune systems". Med. Immunology. 1 (1–2): 17–22.
- Dolotov OV, Karpenko EA, Inozemtseva LS, Seredenina TS, Levitskaya NG, Rozyczka J, Dubynina EV, Novosadova EV, Andreeva LA, Alfeeva LY, Kamensky AA, Grivennikov IA, Myasoedov NF, Engele J (October 2006). "Semax, an analog of ACTH(4-10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus". Brain Res. 1117 (1): 54–60. doi:10.1016/j.brainres.2006.07.108. PMID 16996037. S2CID 27592503.
- Eremin KO, Kudrin VS, Grivennikov IA, Miasoedov NF, Rayevsky KS (2004). "Effects of Semax on dopaminergic and serotoninergic systems of the brain". Dokl. Biol. Sci. 394 (1–6): 1–3. doi:10.1023/b:dobs.0000017114.24474.40. PMID 15088389. S2CID 12955434.
- Eremin KO, Kudrin VS, Saransaari P, Oja SS, Grivennikov IA, Myasoedov NF, Rayevsky KS (December 2005). "Semax, an ACTH(4-10) analogue with nootropic properties, activates dopaminergic and serotoninergic brain systems in rodents". Neurochem. Res. 30 (12): 1493–500. doi:10.1007/s11064-005-8826-8. PMID 16362768. S2CID 38202287.
- Vilenskiĭ DA; Levitskaia NG; Andreeva LA; Alfeeva LIu; Kamenskiĭ AA; Miasoedov NF (June 2007). "[Effects of chronic Semax administration on exploratory activity and emotional reaction in white rats]". Ross Fiziol Zh Im I M Sechenova (in Russian). 93 (6): 661–9. PMID 17850024.
- Yatsenko KA, Glazova NY, Inozemtseva LS, Andreeva LA, Kamensky AA, Grivennikov IA, Levitskaya NG, Dolotov OV, Myasoedov NF (November 2013). "Heptapeptide semax attenuates the effects of chronic unpredictable stress in rats". Dokl. Biol. Sci. 453: 353–7. doi:10.1134/S0012496613060161. PMID 24385169. S2CID 9699654.
- Volodina MA, Sebentsova EA, Glazova NY, Levitskaya NG, Andreeva LA, Manchenko DM, Kamensky AA, Myasoedov NF (March 2012). "Semax attenuates the influence of neonatal maternal deprivation on the behavior of adolescent white rats". Bull. Exp. Biol. Med. 152 (5): 560–3. doi:10.1007/s10517-012-1574-2. PMID 22803132. S2CID 1419653.
- Pae CU (January 2008). "Therapeutic possibility of "Semax" for depression". CNS Spectr. 13 (1): 20–1. doi:10.1017/S1092852900016102. PMID 18204410.
- Bertolini A (March 2012). "Drug-induced activation of the nervous control of inflammation: a novel possibility for the treatment of hypoxic damage". Eur. J. Pharmacol. 679 (1–3): 1–8. doi:10.1016/j.ejphar.2012.01.004. PMID 22293371.
- Kost NV; Sokolov OIu; Gabaeva MV; Grivennikov IA; Andreeva LA; Miasoedov NF; Zozulia AA (2001). "Semax and Selank Inhibit the Enkephalin-Degrading Enzymes of Human Serum". Bioorg. Khim. (in Russian). 27 (3): 180–3. doi:10.1023/A:1011373002885. PMID 11443939. S2CID 26029820.
- Lebedeva, I. S.; Panikratova, Ya R.; Sokolov, O. Yu; Kupriyanov, D. A.; Rumshiskaya, A. D.; Kost, N. V.; Myasoedov, N. F. (September 2018). "Effects of Semax on the Default Mode Network of the Brain". Bulletin of Experimental Biology and Medicine. 165 (5): 653–656. doi:10.1007/s10517-018-4234-3. ISSN 1573-8221. PMID 30225715.
- Kaplan, A., Kochetova, A., Nezavibathko, V., Rjasina, T. and Ashmarin, I. (1996), Synthetic acth analogue semax displays nootropic-like activity in humans. Neurosci. Res. Comm., 19: 115-123. https://doi.org/10.1002/(SICI)1520-6769(199609)19:2<115::AID-NRC171>3.0.CO;2-B
- Gusev, E. I.; Martynov, M. Yu.; Kostenko, E. V.; Petrova, L. V.; Bobyreva, S. N. (2018). "The efficacy of semax in the tretament of patients at different stages of ischemic stroke". Zhurnal nevrologii i psikhiatrii im. S.S. Korsakova. 118 (3): 61. doi:10.17116/jnevro20181183261-68. ISSN 1997-7298.