Modimelanotide
Modimelanotide (INN) (code names AP-214, ABT-719, ZP-1480) is a melanocortinergic peptide drug derived from α-melanocyte-stimulating hormone (α-MSH) which was under development by, at different times, Action Pharma, Abbott Laboratories, AbbVie, and Zealand for the treatment of acute kidney injury.[1][2] It acts as a non-selective melanocortin receptor agonist, with IC50 values of 2.9 nM, 1.9 nM, 3.7 nM, and 110 nM at the MC1, MC3, MC4, and MC5 receptors.[3] Modimelanotide failed clinical trials for acute kidney injury despite showing efficacy in animal models,[4][5] and development was not further pursued.[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | Acetylhexa-L-lysyl-α-MSH |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
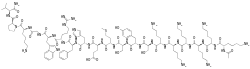
| Formula | C113H181N33O25S |
| Molar mass | 2433.96 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Ramos AM, González-Guerrero C, Sanz A, Sanchez-Niño MD, Rodríguez-Osorio L, Martín-Cleary C, et al. (May 2015). "Designing drugs that combat kidney damage". Expert Opinion on Drug Discovery. 10 (5): 541–56. doi:10.1517/17460441.2015.1033394. PMID 25840605. S2CID 24191324.
- Montero-Melendez T (May 2015). "ACTH: The forgotten therapy". Seminars in Immunology. 27 (3): 216–26. doi:10.1016/j.smim.2015.02.003. PMID 25726511.
- "Renal-Urologic drugs". Drug Data Reports. Prous Science. 29 (1): 42. 2007. ISSN 0379-4121.
- Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, et al. (June 2008). "AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality". Kidney International. 73 (11): 1266–74. doi:10.1038/ki.2008.97. PMC 2398767. PMID 18354376.
- Simmons MN, Subramanian V, Crouzet S, Haber GP, Colombo JR, Ukimura O, et al. (April 2010). "Alpha-melanocyte stimulating hormone analogue AP214 protects against ischemia induced acute kidney injury in a porcine surgical model". The Journal of Urology. 183 (4): 1625–9. doi:10.1016/j.juro.2009.12.007. PMID 20172543.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.