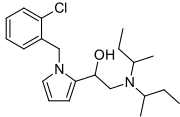
Viminol
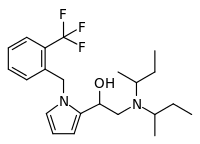
Viminol (marketed under the brandname Dividol) is an opioid analgesic developed by a team at the drug company Zambon in the 1960s.[1] Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Dividol |
| Other names | Dividol, viminolo, diviminol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.040.301 |
| Chemical and physical data | |
| Formula | C21H31ClN2O |
| Molar mass | 362.94 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Viminol has both antitussive (cough suppressing) and analgesic (pain reducing) effects. Viminol has additional effects similar to other opioids including sedation and euphoria. It has six different stereoisomers which have varying properties. Four are inactive, but the 1S-(R,R)-disecbutyl isomer is a μ-opioid full agonist around 5.5 times more potent than morphine and the 1S-(S,S)-disecbutyl isomer is an antagonist.[4][5] Since viminol is supplied as a racemic mixture of isomers, the overall effect is a mixed agonist–antagonist profile similar to that of opioids such as pentazocine, although with somewhat fewer side effects.[6]
Side effects
Side effects are similar to other opioids, and can include:
- Itching
- Nausea
- Sedation
- Respiratory depression - can be potentially life-threatening
However, since viminol is supplied as a racemic mixture of agonist and antagonist isomers, the abuse potential and respiratory depression tends to be less than that of μ-opioid full agonist drugs.
Drug dependence may occur.[7]
Related compounds
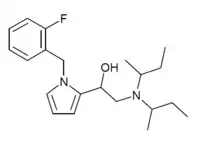
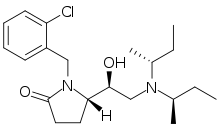
Later work showed that replacing the chlorine atom with an fluorine atom (2F-Viminol) or with a trifluoromethyl group produced a compound with twice the potency and half the acute toxicity.[8] A later team at Zambon found that one isomer of a pyrrolidone analog is 318 times as potent as morphine in its analgesic activity in animal studies.[9] A number of related compounds were also found to be active, allowing a QSAR model to be constructed.
References
- US 3539589, Teotino UM, Bella DD, "1-(α-Pyrryl)-2-amino Ethanols", issued 10 November 1970, assigned to Whitefin Holding SA
- Contri AM (April 1981). "[Chromatographic separation of diastereoisomers of aminoalcohol salts and their densitometric determination]". Il Farmaco; Edizione Pratica (in Italian). 36 (4): 215–22. PMID 6894429.
- Neto JM, Murad JE, Monteiro SS (December 1977). "Psychopharmacological properties of the viminol-p-hydroxybenzoate". Revista Brasileira de Pesquisas Medicas e Biologicas. 10 (6): 361–8. PMID 609773.
- US 3857857, Della D, Bella CV, Monza DC, Tiotino UM, "Stereoisomers of 1(1'(-O-Chlorobenzyl)-2'-Pyrryl)-2-Disec.Butylamino-Ethanol", issued 31 December 1974, assigned to Whitefin Holding SA
- Shook JE, Kallman MJ, Dewey WL (January 1984). "The discriminative stimulus properties of the R2 isomer of viminol". Pharmacology, Biochemistry, and Behavior. 20 (1): 59–62. doi:10.1016/0091-3057(84)90101-1. PMID 6546450. S2CID 11418389.
- Cinelli M, Costa V, Ventresca GP, Lodola E (May 1986). "Viminol R2 analgesic activity in patients with postoperative pain: comparison with pentazocine". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 24 (5): 232–5. PMID 3525423.
- Turkiewicz G, Baltieri DA (2009). "Dependence on Viminol". Journal of Substance Use. 12 (4): 301. doi:10.1080/14659890701237124. S2CID 71184621.
- US 4148907, Conti F, "Stereoisomers of 1-(1'benzyl-2'pyrryl)-2-di-sec.-butylaminoethanol and pharmaceutical compositions comprising same", issued 10 April 1979, assigned to Etablissement Viridis
- US 4960788, Carenzi A, Chiarino D, Bella DD, Grancini GC, Veneziani C, "Pyrrolidone-2 compounds and their use for central analgesic activity", issued 2 October 1990, assigned to Zambon Group S.P.A.
- Napoletano M, Fraire C, Grancini G, Mosotto C, Ricciardi S, Zambon C, et al. (1995). "Stereoselective synthesis and evaluation of all stereoisomers of Z4349, a novel and selective μ-opioid analgesic". Bioorganic & Medicinal Chemistry Letters. 6 (5): 589–592. doi:10.1016/0960-894X(95)00077-7.