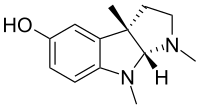
Eseroline
Eseroline is a drug which acts as an opioid agonist.[1] It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible,[2][3] and it produces fairly potent analgesic effects mediated through the μ-opioid receptor.[4] This mixture of activities gives eseroline an unusual pharmacological profile,[5][6] although its uses are limited by side effects such as respiratory depression[7] and neurotoxicity.[8]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Eseroline |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- Fürst S, Friedmann T, Bartolini A, Bartolini R, Aiello-Malmberg P, Galli A, et al. (September 1982). "Direct evidence that eseroline possesses morphine-like effects". European Journal of Pharmacology. 83 (3–4): 233–41. doi:10.1016/0014-2999(82)90256-4. PMID 6293841.
- Jhamandas K, Elliott J, Sutak M (March 1981). "Opiatelike actions of eseroline, an eserine derivative". Canadian Journal of Physiology and Pharmacology. 59 (3): 307–10. doi:10.1139/y81-048. PMID 7194726.
- Galli A, Renzi G, Grazzini E, Bartolini R, Aiello-Malmberg P, Bartolini A (April 1982). "Reversible inhibition of acetylcholinesterase by eseroline, an opioid agonist structurally related to physostigmine (eserine) and morphine". Biochemical Pharmacology. 31 (7): 1233–8. doi:10.1016/0006-2952(82)90009-0. PMID 7092918.
- Agresti A, Buffoni F, Kaufman JJ, Petrongolo C (November 1980). "Structure--activity relationships of eseroline and morphine: ab initio quantum-chemical study of the electrostatic potential and of the interaction energy with water". Molecular Pharmacology. 18 (3): 461–7. PMID 7464812.
- Galli A, Ranaudo E, Giannini L, Costagli C (November 1996). "Reversible inhibition of cholinesterases by opioids: possible pharmacological consequences". The Journal of Pharmacy and Pharmacology. 48 (11): 1164–8. doi:10.1111/j.2042-7158.1996.tb03914.x. PMID 8961166. S2CID 45395195.
- Liu WF (April 1991). "Effect of eseroline on schedule-controlled behavior in the rat". Pharmacology, Biochemistry, and Behavior. 38 (4): 747–51. doi:10.1016/0091-3057(91)90236-U. PMID 1871191. S2CID 12857298.
- Berkenbosch A, Rupreht J, DeGoede J, Olievier CN, Wolsink JG (February 1993). "Effects of eseroline on the ventilatory response to CO2". European Journal of Pharmacology. 232 (1): 21–8. doi:10.1016/0014-2999(93)90723-U. PMID 8458393.
- Somani SM, Kutty RK, Krishna G (October 1990). "Eseroline, a metabolite of physostigmine, induces neuronal cell death". Toxicology and Applied Pharmacology. 106 (1): 28–37. doi:10.1016/0041-008X(90)90102-Z. PMID 2251681.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.