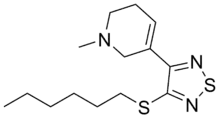
Tazomeline
Tazomeline (LY-287,041) is a drug which acts as a non-selective muscarinic acetylcholine receptor agonist.[1][2] It was in clinical trials for the treatment of cognitive dysfunction such as that seen in Alzheimer's disease and schizophrenia, but development was apparently scrapped for unknown reasons.[1][2][3] Another of the patented uses is for the treatment of "severe painful conditions".
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H23N3S2 |
| Molar mass | 297.48 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Synthesis
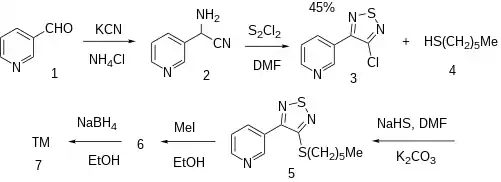
Ex 1: A Strecker type alpha-amino nitrile between nicotinaldehyde, potassium cyanide and ammonium chloride gives amino(pyridin-3-yl)acetonitrile [131988-63-1] (2). The halogenation of this intermediate with sulfur monochloride [10025-67-9] in DMF led to 3-chloro-4-(3-pyridyl)-1,2,5-thiadiazole [131986-28-2] (3).
Ex 64: Thioether formation with 1-Hexanethiol [111-31-9] (4) in the presence of sodium hydrogen sulfide, DMF and K2CO3 gave 3-(4-hexylthio-1,2,5-thiadiazol-3-yl)pyridine, CID:10755149 (5). Alkylation with methyl iodide gives 3-(4-hexylthio-1,2,5-thiadiazol-3-yl)-1-methylpyridinium iodide, CID:19075299 (6). The reduction of the pyridine with sodium borohydride in alcohol gives the tetrahydropyridine and hence completed the synthesis of Tazomeline (7).
See also
References
- Langmead CJ, Watson J, Reavill C (February 2008). "Muscarinic acetylcholine receptors as CNS drug targets". Pharmacology & Therapeutics. 117 (2): 232–43. doi:10.1016/j.pharmthera.2007.09.009. PMID 18082893.
- Amos D Korczyn (October 2000). "Muscarinic M1 Agonists in the Treatment of Alzheimer's Disease". Expert Opinion on Investigational Drugs. 9 (10): 2259–2267(9). doi:10.1517/13543784.9.10.2259. PMID 11060805. S2CID 32214700.
- Mashkovskii MD, Glushkov RG (April 2001). "Drugs for the Treatment of Alzheimer's Disease". Pharmaceutical Chemistry Journal. 35 (4): 179–182. doi:10.1023/A:1010474325601. S2CID 39866378.
- Sauerberg, P., Olesen, P. H., Nielsen, S., Treppendahl, S., Sheardown, M. J., Honore, T., Mitch, C. H., Ward, J. S., Pike, A. J. (June 1992). "Novel functional M1 selective muscarinic agonists. Synthesis and structure-activity relationships of 3-(1,2,5-thiadiazolyl)-1,2,5,6-tetrahydro-1-methylpyridines". Journal of Medicinal Chemistry. 35 (12): 2274–2283. doi:10.1021/jm00090a019. ISSN 1520-4804 0022-2623, 1520-4804.
{{cite journal}}: Check|issn=value (help) - Per Sauerberg & Preben H. Olesen, U.S. Patent 5,712,297 (1998 to Novo Nordisk AS).
- Klosa, J. (1956). "Über einige Kondensationen mit Pyridin- und Chinolinaldehyden". Archiv der Pharmazie. 289 (4): 177–188. doi:10.1002/ardp.19562890402. ISSN 1521-4184 0365-6233, 1521-4184.
{{cite journal}}: Check|issn=value (help)