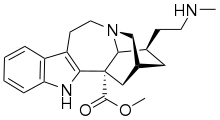
18-Methylaminocoronaridine
(–)-18-Methylaminocoronaridine (18-MAC) is a second generation synthetic derivative of ibogaine developed by the research team led by the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont.[1][2]
 | |
| Identifiers | |
|---|---|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H29N3O2 |
| Molar mass | 367.493 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
See also
References
- Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, et al. (June 2003). "Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents". Journal of Medicinal Chemistry. 46 (13): 2716–30. doi:10.1021/jm020562o. PMID 12801235.
- Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW (May 2004). "Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration". European Journal of Pharmacology. 492 (2–3): 159–67. doi:10.1016/j.ejphar.2004.03.062. PMID 15178360.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.