Pempidine
Pempidine is a ganglion-blocking drug, first reported in 1958 by two research groups working independently, and introduced as an oral treatment for hypertension.[1]
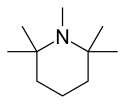 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.102 |
| Chemical and physical data | |
| Formula | C10H21N |
| Molar mass | 155.285 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Pharmacology
Reports on the "classical" pharmacology of pempidine have been published.[2][3] The Spinks group, at ICI, compared pempidine, its N-ethyl analogue, and mecamylamine in considerable detail, with additional data related to several structurally simpler compounds.[2]
Toxicology
LD50 for the HCl salt of pempidine in mice: 74 mg/kg (i.v.); 125 mg/kg (i.p.); 413 mg/kg (p.o.).[2]
Chemistry
Pempidine is an aliphatic, sterically hindered, cyclic, tertiary amine, which is a weak base: in its protonated form it has a pKa of 11.25.[4]
Pempidine is a liquid with a boiling point of 187–188 °C and a density of 0.858 g/cm3.[2]
Two early syntheses of this compound are those of Leonard and Hauck,[5] and Hall.[4] These are very similar in principle: Leonard and Hauck reacted phorone with ammonia, to produce 2,2,6,6-tetramethyl-4-piperidone,[6] which was then reduced by means of the Wolff–Kishner reduction to 2,2,6,6-tetramethylpiperidine; this secondary amine was then N-methylated using methyl iodide and potassium carbonate.[7]
Hall's method involved reacting acetone with ammonia in the presence of calcium chloride to give 2,2,6,6-tetramethyl-4-piperidone, which was then reduced under Wolff-Kishner conditions, followed by N-methylation of the resulting 2,2,6,6-tetramethylpiperidine with methyl p-toluenesulfonate.
References
- Spinks A, Young EH (May 1958). "Polyalkylpiperidines: a new series of ganglion-blocking agents". Nature. 181 (4620): 1397–8. Bibcode:1958Natur.181.1397S. doi:10.1038/1811397a0. S2CID 4196802.
- Spinks A, Young EH, Farrington JA, Dunlop D (December 1958). "The pharmacological actions of pempidine and its ethyl homologue". British Journal of Pharmacology and Chemotherapy. 13 (4): 501–20. doi:10.1111/j.1476-5381.1958.tb00246.x. PMC 1481871. PMID 13618559.
- Muggleton DF, Reading HW (June 1959). "Absorption, metabolism and elimination of pempidine in the rat". British Journal of Pharmacology and Chemotherapy. 14 (2): 202–8. doi:10.1111/j.1476-5381.1959.tb01384.x. PMC 1481796. PMID 13662574.
- Hall HK (1957). "Steric Effects on the Base Strengths of Cyclic Amines". Journal of the American Chemical Society. 79 (20): 5444–5447. doi:10.1021/ja01577a031.
- Leonard NJ, Hauck Jr FP (October 1957). "Unsaturated amines. X. The mercuric acetate route to substituted piperidines, Δ2-tetrahydropyridines and Δ2-tetrahydroanabasines". Journal of the American Chemical Society. 79 (19): 5279–92. doi:10.1021/ja01576a056.
- The "trivial" name of this compound is triacetonamine.
- The boiling point of 147 °C given by these authors for their N,2,2,6,6-pentamethylpiperidine (pempidine) is significantly below the range of ~182–188 °C reported by other chemists.
External links
- Pempidine at the US National Library of Medicine Medical Subject Headings (MeSH)