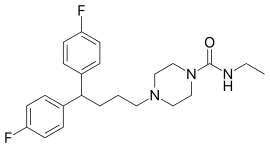
Amperozide
Amperozide is an atypical antipsychotic of the diphenylbutylpiperazine class which acts as an antagonist at the 5-HT2A receptor.[1] It does not block dopamine receptors as with most antipsychotic drugs,[2] but does inhibit dopamine release,[3][4] and alters the firing pattern of dopaminergic neurons.[5] It was investigated for the treatment of schizophrenia in humans,[6] but never adopted clinically. Its main use is instead in veterinary medicine, primarily in intensively farmed pigs, for decreasing aggression and stress and thereby increasing feeding and productivity.[7][8][9]
 | |
| Clinical data | |
|---|---|
| ATCvet code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H29F2N3O |
| Molar mass | 401.502 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
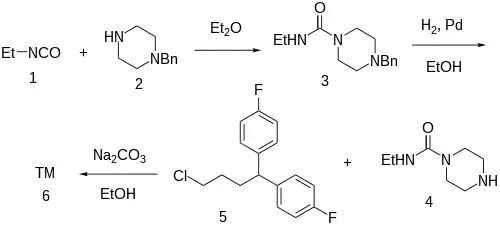
Synthesis
According to Arvid Carlsson, behavioural Stimulation in rat:[10]
The reaction of ethylisocyanate [109-90-0] (1) with N-benzylpiperazine (2) leads to the corresponding urea, CID:47103368 (3). Catalytic hydrogenation cleaves the benzyl protecting group giving N-ethylpiperazine-1-carboxamide [75529-72-5] (4). Alkylation of the unmasked nitrogen with 1,1'-(4-Chlorobutylidene)Bis(4-Fluorobenzene) [3312-04-7] (5) completed the synthesis of amperozide (6).
See also
Other agents in the diphenylbutylpiperazine group include:
References
- Svartengren J, Simonsson P (1990). "Receptor binding properties of amperozide". Pharmacology & Toxicology. 66 Suppl 1: 8–11. doi:10.1111/j.1600-0773.1990.tb01599.x. PMID 2154737.
- Meltzer HY, Zhang Y, Stockmeier CA (May 1992). "Effect of amperozide on rat cortical 5-HT2 and striatal and limbic dopamine D2 receptor occupancy: implications for antipsychotic action". European Journal of Pharmacology. 216 (1): 67–71. doi:10.1016/0014-2999(92)90210-u. PMID 1388121.
- Eriksson E (1990). "Amperozide, a putative anti-psychotic drug: uptake inhibition and release of dopamine in vitro in the rat brain". Life Sciences. 47 (23): 2111–7. doi:10.1016/0024-3205(90)90310-n. PMID 1979998.
- Yamamoto BK, Meltzer HY (October 1992). "The effect of the atypical antipsychotic drug, amperozide, on carrier-mediated striatal dopamine release measured in vivo". The Journal of Pharmacology and Experimental Therapeutics. 263 (1): 180–5. PMID 1403783.
- Grenhoff J, Tung CS, Ugedo L, Svensson TH (1990). "Effects of amperozide, a putative antipsychotic drug, on rat midbrain dopamine neurons recorded in vivo". Pharmacology & Toxicology. 66 Suppl 1: 29–33. doi:10.1111/j.1600-0773.1990.tb01603.x. PMID 2304893.
- Axelsson R, Nilsson A, Christensson E, Björk A (1991). "Effects of amperozide in schizophrenia. An open study of a potent 5-HT2 receptor antagonist". Psychopharmacology. 104 (3): 287–92. doi:10.1007/bf02246025. PMID 1924636.
- Kyriakis SC, Martinsson K, Olsson NG, Bjork A (1990). "Thin sow syndrome (TSS): the effect of amperozide". The British Veterinary Journal. 146 (5): 463–7. doi:10.1016/0007-1935(90)90036-3. PMID 2224491.
- Kyriakis SC, Olsson NG, Martinsson K, Björk AK (September 1991). "Observations on the action of amperozide: are there social influences on sow-litter productivity?". Research in Veterinary Science. 51 (2): 169–73. doi:10.1016/0034-5288(91)90008-C. PMID 1788479.
- Papp I, Waller C, Biro O (October 1996). "[Practical experiences in the therapy of postweaning edema disease in piglets]". Berliner und Munchener Tierarztliche Wochenschrift. 109 (10): 385–7. PMID 8999770.
- Waters, N., Pettersson, G., Carlsson, A., Svensson, K. (August 1989). "The putatively antipsychotic agent amperozide produces behavioural stimulation in the rat: A behavioural and biochemical characterization". Naunyn-Schmiedeberg’s Archives of Pharmacology. 340 (2). doi:10.1007/BF00168964. Retrieved 22 October 2022.
- DE2941880 idem Anders K. K. Bjork, Knut G. Olsson, Aina L. Abramo, Erik G. Christensson, U.S. Patent 4,447,433 (1984 to Ab Ferrosan).
- Anders K. K. Bjork, Knut G. Olsson, Aina L. Abramo, Erik G. Christensson, U.S. Patent 4,385,057 (1983 to Ab Ferrosan).