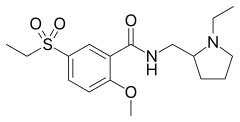
Sultopride
Sultopride (trade names Barnetil, Barnotil, Topral) is an atypical antipsychotic of the benzamide chemical class used in Europe, Japan, and Hong Kong for the treatment of schizophrenia.[1][2][3] It was launched by Sanofi-Aventis in 1976.[1] Sultopride acts as a selective D2 and D3 receptor antagonist.[4] It has also been shown to have clinically relevant affinity for the GHB receptor as well, a property it shares in common with amisulpride and sulpiride.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Barnetil, Barnotil, Topral |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3–5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.293 |
| Chemical and physical data | |
| Formula | C17H26N2O4S |
| Molar mass | 354.47 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- José Miguel Vela; Helmut Buschmann; Jörg Holenz; Antonio Párraga; Antoni Torrens (2007). Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application. Weinheim: Wiley-VCH. ISBN 3-527-31058-4.
- Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- European Drug Index, 4th Edition. Boca Raton: CRC Press. 1998. ISBN 3-7692-2114-1.
- Burstein, E. S.; Ma, J; Wong, S; Gao, Y; Pham, E; Knapp, AE; Nash, NR; Olsson, R; Davis, RE (2005). "Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist". The Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1278–87. doi:10.1124/jpet.105.092155. PMID 16135699.
- Maitre M, Ratomponirina C, Gobaille S, Hodé Y, Hechler V (1994). "Displacement of [3H] gamma-hydroxybutyrate binding by benzamide neuroleptics and prochlorperazine but not by other antipsychotics". Eur J Pharmacol. 256 (2): 211–4. doi:10.1016/0014-2999(94)90248-8. PMID 7914168.
- Burstein, E. S.; Ma, J.; Wong, S.; Gao, Y.; Pham, E.; Knapp, A. E.; Nash, N. R.; Olsson, R.; Davis, R. E.; Hacksell, U.; Weiner, D. M. (December 2005). "Intrinsic Efficacy of Antipsychotics at Human D 2 , D 3 , and D 4 Dopamine Receptors: Identification of the Clozapine Metabolite N -Desmethylclozapine as a D 2 /D 3 Partial Agonist". Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1278–1287. doi:10.1124/jpet.105.092155. ISSN 0022-3565.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.