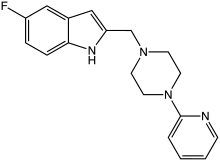
CP-226,269
CP-226,269 is a drug which acts as a dopamine agonist selective for the D4 subtype, which is used for researching the role of D4 receptors in the brain.[1][2]
 | |
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H19FN4 |
| Molar mass | 310.376 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
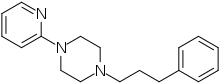
The piperazine used has dual use in the synthesis of ABT-724, ABT-670, Azaperone, MLS 1547 [315698-36-3], Revenast [85673-87-6], UMB38 & XH-148.
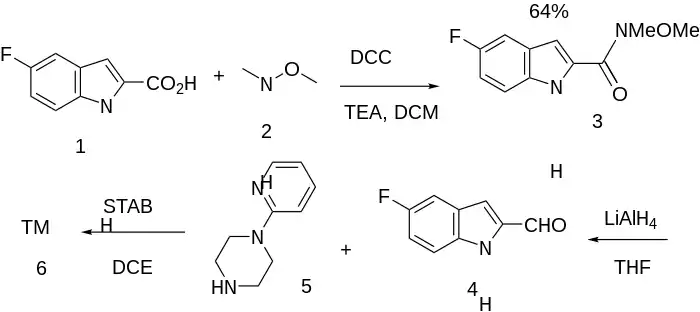
Patent (Ex 15):[3]
Weinreb ketone synthesis between 5-Fluoroindole-2-Carboxylic acid [399-76-8] (1) and N,O-Dimethylhydroxylamine Fb: [1117-97-1] Hcl: [6638-79-5] (2) gives the Weinreb–Nahm amide, 5-Fluoro-N-Methoxy-N-Methyl-Indole-2-Carboxamide, CID:23003585 (3). This intermediate is further reduced giving 5-Fluoro-Indole-2-Carbaldehyde [220943-23-7] (4). Reductive amination with 1-(2-Pyridyl)Piperazine [34803-66-2] (5) completed the synthesis CP-226,269 (6).
References
- Sharma A, Kramer ML, Wick PF, Liu D, Chari S, Shim S, Tan W, Ouellette D, Nagata M, DuRand CJ, Kotb M, Deth RC (May 1999). "D4 dopamine receptor-mediated phospholipid methylation and its implications for mental illnesses such as schizophrenia". Molecular Psychiatry. 4 (3): 235–46. doi:10.1038/sj.mp.4000522. PMID 10395213.
- Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, Drescher K, Fox GB, Decker MW, Rueter LE (July 2005). "Antidepressant-like effect of D(2/3) receptor-, but not D(4) receptor-activation in the rat forced swim test". Neuropsychopharmacology. 30 (7): 1257–68. doi:10.1038/sj.npp.1300677. PMID 15688083.
- Anton Franz Josef Fliri, et al. WO 1999009025 (Pfizer Products Inc).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.