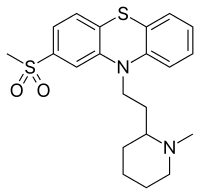
Sulforidazine
Sulforidazine (Imagotan, Psychoson, Inofal) a typical antipsychotic and a metabolite of thioridazine; it and mesoridazine are more potent than the parent compound, whose pharmacological effects are believed by some to be largely due to its metabolism into sulforidazine and mesoridazine.[1]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.035.274 |
| Chemical and physical data | |
| Formula | C21H26N2O2S2 |
| Molar mass | 402.57 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
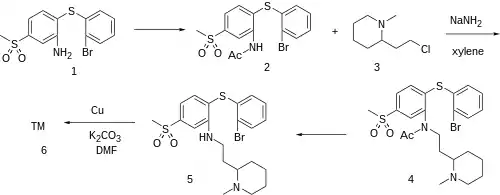
Synthesis
2-bromo-2'-amino-4'-methylsulphonyl-diphenyl Sulphide, CID:43448246 (1) 2-bromo-2'-acetamino-4'-methylsulphonyl diphenylsulphide (2) 2-(2-Chloroethyl)-1-Methylpiperidine [50846-01-0] (3)
References
- Niedzwiecki DM, Mailman RB, Cubeddu LX (March 1984). "Greater potency of mesoridazine and sulforidazine compared with the parent compound, thioridazine, on striatal dopamine autoreceptors". Journal of Pharmacology and Experimental Therapeutics. 228 (3): 636–9. PMID 6707914.
- Morrow, Ryan J.; Millership, Jeff S.; Collier, Paul S. (2005). "Facile Syntheses of the Three Major Metabolites of Thioridazine". Helvetica Chimica Acta. 88 (5): 962–967. doi:10.1002/hlca.200590089.
- FR1363683 idem Bruschweiler Conrad, Schwarb Gustav, Winkler Hans, Renz Jany, U.S. Patent 3,314,948 (1967 to Sandoz Ltd).
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
Acetylcholine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.