Nizatidine
Nizatidine is a histamine H2 receptor antagonist that inhibits stomach acid production, and is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease.[1]
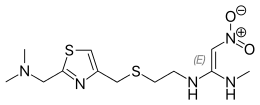 | |
| Clinical data | |
|---|---|
| Trade names | Axid, Tazac |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >70% |
| Protein binding | 35% |
| Metabolism | Liver |
| Elimination half-life | 1–2 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.155.683 |
| Chemical and physical data | |
| Formula | C12H21N5O2S2 |
| Molar mass | 331.45 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was patented in 1980 and approved for medical use in 1988.[2][3] It was developed by Eli Lilly. Brand names include Tazac and Axid.
Medical use
Nizatidine is used to treat duodenal ulcers, gastric ulcers, and gastroesophageal reflux disease (GERD/GORD), and to prevent stress ulcers.[4]
Adverse effects
Side effects are uncommon, usually minor, and include diarrhea, constipation, fatigue, drowsiness, headache, and muscle aches.[4]
History and development
Nizatidine was developed by Eli Lilly, and was first marketed in 1988.[2] It is considered to be equipotent with ranitidine and differs by the substitution of a thiazole ring in place of the furan ring in ranitidine. In September 2000, Eli Lilly announced they would sell the sales and marketing rights for Axid to Reliant Pharmaceuticals.[5] Subsequently, Reliant developed the oral solution of Axid, marketing this in 2004, after gaining approval from the U.S. Food and Drug Administration (FDA).[6] However, a year later, they sold rights of the Axid Oral Solution (including the issued patent[7] protecting the product) to Braintree Laboratories.[8]
Nizatidine proved to be the last new histamine H2 receptor antagonist introduced prior to the advent of proton pump inhibitors.
Axid (nizatidine) drug recalled due to presence of NDMA.
See also
- Famotidine (Pepcid) — another popular H2 receptor antagonist
References
- Romero M, Franzosi MG (1989). "[Nizatidine]". Medicina (Florence, Italy) (in Italian). 9 (1): 93–6. PMID 2567957.
- "Nizatidine: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 20 March 2020.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 44. ISBN 9783527607495.
- "Nizatidine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. NCBI Bookshelf. 25 January 2018. PMID 31643707. NBK548387. Retrieved 19 March 2020.
- "Eli Lilly and Company and Reliant Pharmaceuticals Announce Agreement for U.S. Sales and Marketing Rights to Axid(R)". High Beam Encyclopedia. 7 September 2000. Archived from the original on May 26, 2008.
- "Reliant Pharmaceuticals to Launch AxidŽ Oral Solution". Reliant Pharmaceuticals, LLC. 26 July 2004.
- US 6930119, Bobotas G, Fawzy AA, "Liquid pharmaceutical composition", issued 24 June 2005, assigned to Reliant Pharmaceuticals, LLC
- "Reliant Pharmaceuticals Announces the Sale of Axid® Oral Solution to Braintree Laboratories". Reliant Pharmaceuticals, LLC. Archived from the original on August 14, 2007.
External links
- "Nizatidine". Drug Information Portal. U.S. National Library of Medicine.