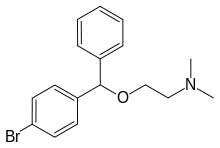
Bromazine
Bromazine, sold under the brand names Ambodryl, Ambrodil, and Deserol among others, also known as bromodiphenhydramine, is an antihistamine and anticholinergic medication of the ethanolamine class.[1][2][3][4][5] It is an analogue of diphenhydramine with a bromine substitution on one of the phenyl rings.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Ambodryl, Ambrodil, Deserol |
| Other names | Bromodiphenhydramine; Bromdiphenhydramine |
| MedlinePlus | a682065 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 96% |
| Metabolism | Mostly hepatic (CYP-mediated), also renal |
| Elimination half-life | 1 to 4 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.854 |
| Chemical and physical data | |
| Formula | C17H20BrNO |
| Molar mass | 334.257 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
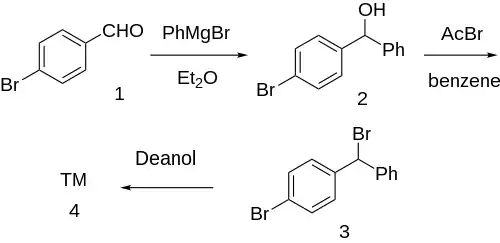
Synthesis
Grignard reaction between phenylmagnesium bromide and para-bromobenzaldehyde [1122-91-4] (1) gives p-bromobenzhydrol [29334-16-5] (2). Halogenation with acetyl bromide in benzene solvent gives p-bromo-benzhydrylbromide [18066-89-2] (3). Finally, etherification with deanol completed the synthesis of Bromazine (4).
References
- J. Elks, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 177–. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 134–. ISBN 978-3-88763-075-1.
- Baker CE (1974). Physicians' Desk Reference (28 ed.). Oradell, NJ 07649: Medical Economics Company. pp. 1076, 1081.
{{cite book}}: CS1 maint: location (link) - Kalpaklioglu F, Baccioglu A (2012). "Efficacy and safety of H1-antihistamines: an update". Anti-Inflamm Anti-Allergy Agents Med Chem. 11 (3): 230–7. doi:10.2174/1871523011202030230. PMID 23173575.
- Maclaren WR, Bruff WC, Eisenberg BC, Weiner H, Martin WH (1955). "A clinical comparison of carbinoxamine maleate, tripelennamine hydrochloride, and bromodiphenhydramine hydrochloride in treating allergic symptoms". Annals of Allergy. 13 (3): 307–12. PMID 14377226.
- Ahmadi, Abbas; Khalili, Mohsen; Hajikhani, Ramin; Safari, Narjes; Nahri-Niknafs, Babak (2011). "Anti-inflammatory effects of two new methyl and morpholine derivatives of diphenhydramine on rats". Medicinal Chemistry Research. 21 (11): 3532–3540. doi:10.1007/s00044-011-9891-y.
- Jr George Rieveschl, US2527963 (1950 to Parke Davis & Co).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.