Pitolisant
Pitolisant, sold under the brand name Wakix among others, is a medication for the treatment of excessive daytime sleepiness in adults with narcolepsy.[2] It is a histamine 3 (H3) receptor antagonist/inverse agonist.[2] It represents the first commercially available medication in its class.[5] Pitolisant enhances the activity of histaminergic neurons in the brain that function to improve a person's wakefulness.[6]
 | |
| Clinical data | |
|---|---|
| Trade names | Wakix, Ozawade |
| Other names | Tiprolisant; Ciproxidine; BF2.649 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619055 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Histamine H3 receptor inverse agonists |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
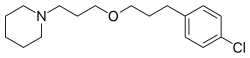
| Formula | C17H26ClNO |
| Molar mass | 295.85 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The most common side effects include difficulty sleeping, nausea, and feeling worried.[7]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[8]
Medical uses
Pitolisant (Wakix) is used in adults for the treatment of excessive daytime sleepiness.[2][3] Narcolepsy is a sleep problem that is characterized by an irresistible urge to sleep and disturbed nighttime sleep, while cataplexy refers to attacks of severe muscle weakness that cause a person to collapse.[3] Pitolisant (Ozawade) is indicated to improve wakefulness and reduce excessive daytime sleepiness in adults with obstructive sleep apnea.[4]
Side effects
The most common side effects include insomnia (difficulty sleeping), headache, nausea (feeling sick), anxiety, irritability, dizziness, depression, tremor, sleep disorders, tiredness, vomiting, vertigo (a spinning sensation) and dyspepsia (heartburn).[3] Serious but rare side effects are abnormal loss of weight and spontaneous abortion.[3]
History
Pitolisant was y Jean-Charles Schwartz, Walter Schunack, and colleagues after the former discovered the H3 receptor.[9] It was the first H3 receptor inverse agonist to be tested in humans or introduced for clinical use.[9] It is marketed in the European Union by Bioprojet Pharma.[3] It was approved for medical use in the European Union in March 2016.[3]
The FDA approved pitolisant for excessive daytime sleepiness in participants with narcolepsy based primarily on evidence from two trials (Trial 1/NCT01067222, Trial 2/NCT01638403).[7] An additional trial (Trial 3/NCT01800045), in which participants with a different type of narcolepsy were exposed to the same dose of pitolisant, was used to add data for evaluation of side effects.[7] The trials were conducted in Europe and South America.[7]
The two primary trials enrolled adults with narcolepsy and excessive daytime sleepiness.[7] Participants received pitolisant, placebo, or an approved drug for narcolepsy for eight weeks.[7] For participants receiving pitolisant, the dose could be increased during the first three weeks but had to remain the same for the next five weeks.[7] Neither the participants nor the healthcare providers knew which treatment was being given during the trial.[7]
The benefit of pitolisant was evaluated by comparing changes in daytime sleepiness during the trial between pitolisant- and placebo-treated participants.[7] To measure the daytime sleepiness, the investigators used a scale called the Epworth Sleepiness Scale (ESS).[7] The ESS asks participants to rate the likelihood that they would fall asleep while doing eight daily activities (such as sitting and reading or watching television).[7] Participants rate each item from zero (would never doze) to three (high chance of dozing).[7]
Pitolisant was approved by the U.S. Food and Drug Administration (FDA) in August 2019.[7] It was granted orphan drug designation for the treatment of narcolepsy,[10] fast track designation for the treatment of excessive daytime sleepiness and cataplexy in people with narcolepsy, and breakthrough therapy designation for the treatment of cataplexy in people with narcolepsy.[11]
References
- "Summary Basis of Decision (SBD) for Wakix". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- "Wakix- pitolisant hydrochloride tablet, film coated". DailyMed. 6 November 2019. Retrieved 18 August 2020.
- "Wakix EPAR". European Medicines Agency (EMA). Retrieved 18 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Ozawade EPAR". European Medicines Agency (EMA). Retrieved 15 October 2021.
- "FDA Approves Pitolisant for Daytime Sleepiness in Patients with Narcolepsy". Pharmacy Times. Retrieved 18 August 2020.
- Syed YY (20 July 2016). "Pitolisant: First Global Approval". Drugs. 76 (13): 1313–1318. doi:10.1007/s40265-016-0620-1. PMID 27438291. S2CID 42684839.
- "Drug Trials Snapshots: Wakix". U.S. Food and Drug Administration (FDA). 14 August 2019. Retrieved 18 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Retrieved 15 September 2020.
- Schwartz JC (2011). "The histamine H3 receptor: from discovery to clinical trials with pitolisant". Br. J. Pharmacol. 163 (4): 713–21. doi:10.1111/j.1476-5381.2011.01286.x. PMC 3111674. PMID 21615387.
- "Pitolisant Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 17 May 2010. Retrieved 25 May 2021.
- "Harmony's pitolisant granted breakthrough and fast track designations". Pharma Business International. 22 May 2018. Retrieved 25 May 2021.
External links
- "Pitolisant". Drug Information Portal. U.S. National Library of Medicine.
- "Ciproxidine". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT01067222 for "Efficacy and Safety Study of BF2.649 in the Treatment of Excessive Daytime Sleepiness in Narcolepsy (Harmony1)" at ClinicalTrials.gov
- Clinical trial number NCT01638403 for "Effects of BF2.649 in the Treatment of Excessive Daytime Sleepiness in Narcolepsy." at ClinicalTrials.gov