Morazone
Morazone (Novartrina, Orsimon, Rosimon-Neu, Tarcuzate) is a nonsteroidal anti-inflammatory drug (NSAID), originally developed by the German pharmaceutical company Ravensberg in the 1950s, which is used as an analgesic.[1][2][3] It produces phenmetrazine as a major metabolite and has been reported to have been abused as a recreational drug in the past.[4][5][6][7]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, SC, IM[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ECHA InfoCard | 100.026.771 |
| Chemical and physical data | |
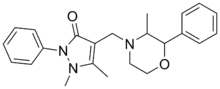
| Formula | C23H27N3O2 |
| Molar mass | 377.488 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
See also
References
- Seyffart G (1991). Drug dosage in Renal Insufficiency. Boston: Kluwer Academic Publishers. p. 399. ISBN 978-0-7923-0964-2.
- US patent 2943022, Siemer, H. & Doppstadt, A., "Substituted 1-phenyl-2,3-dimethyl-4-morpholino methyl pyrazolone-(5) Compounds and Process of Making Same", issued 1960-06-28, assigned to Ravensberg
- Buckingham J, ed. (1996). Dictionary of Organic Compounds. Vol. 7. London: Chapman & Hall. p. 4659. ISBN 978-0-412-54090-5.
- Bohn G, Rücker G, Kröger H (June 1976). "[Investigations of the decomposition and detection of morazone by thin-layer- and gas-liquid-chromatography]". Archives of Toxicology. 35 (3): 213–20. doi:10.1007/bf00293569. PMID 989292. S2CID 23956851.
- Neugebauer M (1984). "Some new urinary metabolites of famprofazone and morazone in man". Journal of Pharmaceutical and Biomedical Analysis. 2 (1): 53–60. doi:10.1016/0731-7085(84)80089-8. PMID 16867765.
- Kingreen JC, Breger G (May 1984). "[Pellagra in morazone abuse]". Zeitschrift für Hautkrankheiten. 59 (9): 573–7. PMID 6145264.
- Daunderer M, Janzen W (1972). "[ROSIMON-NEU--a non-prescription analgesic on the adolescent drug scene]". Beiträge zur Gerichtlichen Medizin. 29: 138–43. PMID 5081964.
| Adamantanes | |
|---|---|
| Adenosine antagonists |
|
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics |
|
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines |
|
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines |
|
| Others |
|
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.