8-OH-DPAT
8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to be discovered.
 | |
| Names | |
|---|---|
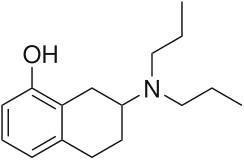
| Systematic IUPAC name
7-(Dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | 8-OH-DPAT |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 8-Hydroxy-2-(di-n-propylamino)tetralin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C16H25NO |
| Molar mass | 247.382 g·mol−1 |
| log P | 3.711 |
| Acidity (pKa) | 10.539 |
| Basicity (pKb) | 3.458 |
| Pharmacology | |
| Pharmacokinetics: | |
| 1.5 hours | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Originally believed to be selective for the 5-HT1A receptor, 8-OH-DPAT was later found to act as a 5-HT7 receptor agonist and serotonin reuptake inhibitor/releasing agent as well.[2][3][4][5][6]
In animal studies, 8-OH-DPAT has been shown to possess antidepressant,[7] anxiolytic,[8] serenic,[9] anorectic,[10] antiemetic,[11] hypothermic,[12] hypotensive,[13] bradycardic,[13] hyperventilative,[14][15][16] and analgesic effects.[17]
See also
- 5-OH-DPAT
- 7-OH-DPAT
- Bay R 1531
- MDAT
- UH-301
References
- "8-hydroxy-2-(di-n-propylamino)tetralin - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Larsson LG; Rényi L; Ross SB; Svensson B; Angeby-Möller K (February 1990). "Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT". Neuropharmacology. 29 (2): 85–91. doi:10.1016/0028-3908(90)90047-U. PMID 1691832. S2CID 39066002.
- Sprouse J; Reynolds L; Li X; Braselton J; Schmidt A (January 2004). "8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production". Neuropharmacology. 46 (1): 52–62. doi:10.1016/j.neuropharm.2003.08.007. PMID 14654097. S2CID 41623573.
- "IUPHAR DATABASE - 5-Hydroxytryptamine receptors - 5-HT7".
- Assié MB; Koek W (November 1996). "Possible in vivo 5-HT reuptake blocking properties of 8-OH-DPAT assessed by measuring hippocampal extracellular 5-HT using microdialysis in rats". British Journal of Pharmacology. 119 (5): 845–50. doi:10.1111/j.1476-5381.1996.tb15749.x. PMC 1915946. PMID 8922730.
- Wölfel R; Graefe KH (February 1992). "Evidence for various tryptamines and related compounds acting as substrates of the platelet 5-hydroxytryptamine transporter". Naunyn-Schmiedeberg's Archives of Pharmacology. 345 (2): 129–36. doi:10.1007/BF00165727. PMID 1570019. S2CID 2984583.
- Luscombe GP; Martin KF; Hutchins LJ; Gosden J; Heal DJ (March 1993). "Mediation of the antidepressant-like effect of 8-OH-DPAT in mice by postsynaptic 5-HT1A receptors". British Journal of Pharmacology. 108 (3): 669–77. doi:10.1111/j.1476-5381.1993.tb12859.x. PMC 1908013. PMID 8467355.
- Schreiber R; De Vry J (November 1993). "Neuronal circuits involved in the anxiolytic effects of the 5-HT1A receptor agonists 8-OH-DPAT ipsapirone and buspirone in the rat". European Journal of Pharmacology. 249 (3): 341–51. doi:10.1016/0014-2999(93)90531-L. PMID 7904566.
- de Boer SF; Koolhaas JM (December 2005). "5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis". European Journal of Pharmacology. 526 (1–3): 125–39. doi:10.1016/j.ejphar.2005.09.065. PMID 16310183.
- Dourish CT; Hutson PH; Curzon G (October 1985). "Characteristics of feeding induced by the serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT)". Brain Research Bulletin. 15 (4): 377–84. doi:10.1016/0361-9230(85)90005-X. PMID 2933126. S2CID 11047288.
- Lucot JB (February 1994). "Antiemetic effects of flesinoxan in cats: comparisons with 8-hydroxy-2-(di-n-propylamino)tetralin". European Journal of Pharmacology. 253 (1–2): 53–60. doi:10.1016/0014-2999(94)90756-0. PMID 8013549.
- O'Connell MT; Sarna GS; Curzon G (July 1992). "Evidence for postsynaptic mediation of the hypothermic effect of 5-HT1A receptor activation". British Journal of Pharmacology. 106 (3): 603–9. doi:10.1111/j.1476-5381.1992.tb14382.x. PMC 1907559. PMID 1387027.
- Fozard JR; Mir AK; Middlemiss DN (March 1987). "Cardiovascular response to 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) in the rat: site of action and pharmacological analysis". Journal of Cardiovascular Pharmacology. 9 (3): 328–47. doi:10.1097/00005344-198703000-00010. PMID 2437400. S2CID 24327371.
- Sahibzada N; Ferreira M; Wasserman AM; Taveira-DaSilva AM; Gillis RA (February 2000). "Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors". The Journal of Pharmacology and Experimental Therapeutics. 292 (2): 704–13. PMID 10640309.
- Meyer LC; Fuller A; Mitchell D (February 2006). "Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 290 (2): R405–13. doi:10.1152/ajpregu.00440.2005. PMID 16166206.
- Guenther U; Manzke T; Wrigge H; Dutschmann M; Zinserling J; Putensen C; Hoeft A (April 2009). "The counteraction of opioid-induced ventilatory depression by the serotonin 1A-agonist 8-OH-DPAT does not antagonize antinociception in rats in situ and in vivo". Anesthesia and Analgesia. 108 (4): 1169–76. doi:10.1213/ane.0b013e318198f828. PMID 19299781. S2CID 25951835.
- Xu W; Qiu XC; Han JS (June 1994). "Serotonin receptor subtypes in spinal antinociception in the rat". The Journal of Pharmacology and Experimental Therapeutics. 269 (3): 1182–9. PMID 8014862.
External links
- Yves Aubert, Thesis, Leiden University. (Dec 11, 2012) Sex, aggression and pair-bond: a study on the serotonergic regulation of female sexual function in the marmoset monkey
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.