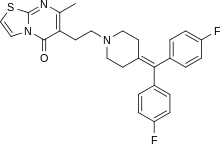
Ritanserin
Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent.[1][2][3] It was never marketed for medical use due to safety problems but has been used in scientific research to study the serotonin system.[2][4]
 | |
| Clinical data | |
|---|---|
| Other names | R-55667; R55667; Tiserton |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.772 |
| Chemical and physical data | |
| Formula | C27H25F2N3OS |
| Molar mass | 477.57 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pharmacology
Pharmacodynamics
Ritanserin acts as a selective 5-HT2A (Ki = 0.45 nM) and 5-HT2C receptor (Ki = 0.71 nM) antagonist.[5][6] It has relatively low affinity for the H1, D2, α1-adrenergic, and α2-adrenergic receptors (39-, 77-, 107-, and 166-fold lower relative to 5-HT2A, respectively).[6] The affinity of ritanserin for the 5-HT1A receptor is less than 1 μM.[6] In addition to its affinity for the 5-HT2A and 5-HT2C receptors, ritanserin also binds to and antagonizes the 5-HT1D, 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7 receptors.[7]
History
The atypical antipsychotic risperidone was developed from ritanserin.[8]
Society and culture
Research
Ritanserin was tested in clinical trials for depression,[3] anxiety, schizophrenia,[5] and migraine.[11] It was also found to improve sleep in human volunteers.[4]
Synthesis
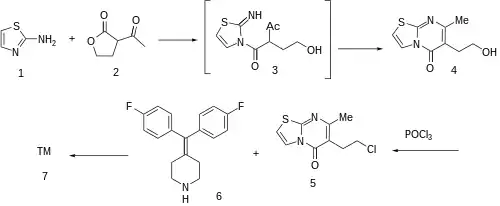
Aminothiazole (2-thiazolamine) (1) is condensed with 2-acetylbutyrolactone [517-23-7] (2) under DS-trap until the water has separated. Condensation of this β-keto lactone can be visualized to involve initial attack on the reactive butyrolactone by the primary nitrogen; cyclodehydration of that hypothetical intermediate 3 gives 6-(2-hydroxyethyl)-7-methyl-[1,3]thiazolo[3,2-a]pyrimidin-5-one, CID:82612453 (4). Halogenation of the terminal alcohol with phosphorus oxychloride then yields 6-(2-chloroethyl)- 7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one, [86488-00-8] (5). Alkylation with 4-(bis(4-fluorophenyl)methylene)piperidine, [58113-36-3] (6) would complete the synthesis of ritanserin (7).
See also
- Ketanserin
- Setoperone
References
- J. Elks, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 411. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- Dr. Ian Morton; I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 249–. ISBN 978-0-7514-0499-9.
- Jonathan Edward Alpert (14 May 2014). Jonathan Edward Alpert; Maurizio Fava (eds.). Handbook of Chronic Depression: Diagnosis and Therapeutic Management. CRC Press. pp. 117–. ISBN 978-0-8247-5660-4.
- Atkin T, Comai S, Gobbi G (April 2018). "Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery". Pharmacol Rev. 70 (2): 197–245. doi:10.1124/pr.117.014381. PMID 29487083. S2CID 3578916.
- Akhondzadeh S, Malek-Hosseini M, Ghoreishi A, Raznahan M, Rezazadeh SA (September 2008). "Effect of ritanserin, a 5HT2A/2C antagonist, on negative symptoms of schizophrenia: A double-blind randomized placebo-controlled study". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 32 (8): 1879–83. doi:10.1016/j.pnpbp.2008.08.020. PMID 18801405. S2CID 12270281.
- Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PF, Laduron PM (1985). "Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist". Mol Pharmacol. 27 (6): 600–11. PMID 2860558.
- Harmful Non-Indigenous Species in the United States. DIANE Publishing. 1 February 1993. pp. 361–. ISBN 978-0-7881-0441-1.
- Bentham Science Publishers (May 1994). Current Medicinal Chemistry. Bentham Science Publishers. pp. 52–.
- "Micromedex Products: Please Login".
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. ISBN 978-3-88763-075-1.
- Nappi, G; Sandrini, G; Granella, F; Ruiz, L; Cerutti, G; Facchinetti, F; Blandini, F; Manzoni, GC (June 1990). "A new 5-HT2 antagonist (ritanserin) in the treatment of chronic headache with depression. A double-blind study vs amitriptyline". Headache. 30 (7): 439–44. doi:10.1111/j.1526-4610.1990.hed3007439.x. hdl:11380/740716. PMID 2119355. S2CID 25781431.
- Prous, J.; Castaner, J.; Ritanserin. Drugs Fut 1986, 11, 5, 391.
- Ludo E. J. Kennis, Jan Vandenberk, Josephus C. Mertens, U.S. Patent 4,485,107 (1984 to Janssen Pharmaceutica N.V.)
- Ludo Edmond Josephine Kennis, et al. EP 0110435 (1989 to Janssen Pharmaceutica NV).