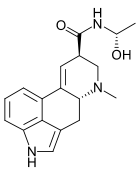
Lysergic acid hydroxyethylamide
D-Lysergic acid α-hydroxyethylamide (LSH, LAH), also known as D-lysergic acid methyl carbinolamide, is an alkaloid of the ergoline family, believed to be present in small amounts in various species in the Convolvulaceae (morning glory), as well as some species of fungi.
 | |
| Clinical data | |
|---|---|
| Other names | D-lysergic acid methyl carbinolamide |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.079 |
| Chemical and physical data | |
| Formula | C18H21N3O2 |
| Molar mass | 311.385 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chemistry
The structure is similar to LSD, with the N,N- diethylamide group replaced by an N- (1- hydroxyethyl)amide in D-lysergic acid α-hydroxyethylamide.
Pharmacology
The intravenous LD50 of the new alkaloid was approximately 150mg/kg for mice and 0.75 mg/kg for rabbits/ Before death, the mice showed periodic convulsions of a clonic type, erection of the hairs and excitability. At doses of 50-100mg/kg it produced only this peculiar symptomatology: the mice stood upright and pressed on each other's noses and chattered their teeth. In rabbits, and injection into the ear vein in doses of 0.1-1mg/kg produced dilatation of the pupil, excitability or convulsions of a clinic type. The ears became pale and cold with intense vasoconstriction. The toxicity of the alkaloid to rabbits seemed to depend on its power of raising the body-temperature in this species. In rabbits the similarity between the effects of the new alkaloid and ergometrine were particularly striking, but ergometrine was less toxic to rabbits (the approximate intravenous LD50 of ergometrine was 3.5mg/kg).
D-lysergic acid methyl carbinolamide induced, in low concentrations (minimum active concentration 0.1-1μg/ml), a contracture in the isolated uterus of the virgen guinea pig. There was a satisfactory dose–response relationship. This contracture was very similar to that produced by ergometrine maleate, which, however, was 1-2 times more potent. On the rabbit uterus in situ both alkaloids produced a prompt contraction and increased rhythmic activity of the uterus. For ergometrine the minimum active dose by intravenous route was 0.1-0.3 mg/kg and for the new alkaloid 0.2-0.5mg/kgm. The actions of both alkaloids lasted some minutes, and owing to the favourable circumstance that the interference between the effects of the two alkaloids was negligible, it was possible to test them on the same preparation. Ergometrine was 1-2 times more potent than the new alkaloid.
On the isolated seminal vesicles of the guinea pig, the new alkaloid was approximately 200 times less potent than ergotamine tartrate as an adrenergic blocking drug. Rabbits anaethetized with urethane supported doses of D-lysergic acid methyl carbinolamide which would have killed unanaesthetized animals. Rapid intravenous injections of small doses (0.1-0.2mg/kg) of the new alkaloid caused an evanescent decrease or a small increase of blood pressure; with higher doses (0.3/0.5mg/kg and more) the blood pressure increased moderately without showing any dose/response relationship.
Ergometrine maleate seemed to be less active on blood pressure, and there was no significant change of blood pressure with 0.3-0.5 mg/kg. The new alkaloid was without effect, when given in small doses, on the blood pressure of cats anaethetized with chloralose. Higher intravenous doses (0.1-0.3mg/kg) caused a sustained hypotension of long duration and a moderate decrease of heart-rate. The respiration of rabbits and cats was depressed by small doses of the new alkaloid; cats seemed to be less resistant than rabbits. In cats, 0.01mg/kg of the new alkaloid caused broncho-constriction and contractions of the nictitating membrane of long duration. The new alkaloid have no action on isolated rabbit auricles at doses up to 100μg/ml.
In summing up, the new naturally occurring alkaloid D-lysergic acid methyl carbinolamide has powerful ergometrine-like oxytocic action and weak ergotamine-like adrenergic blocking actions. It must be included, on the basis of pharmacological evidence, in the ergometrine group of ergot alkaloids. Ergometrine, however, is less toxic and more active than the new alkaloid. Results suggest that it could have a lysergic acid diethylamide-like activity, but this hypothesis must be checked by experiments on humans.[2]
— Glasser, A.
Effects
One of the alkaloids in the seeds of Rivea corymbosa (Ololiuhqui), Argyreia nervosa (Hawaiian Baby Woodrose), and Ipomoea violacea (Tlitliltzin) are ergine (LSA) and isoergine (its epimer).[3] The human activity of D-lysergic acid α-hydroxyethylamide is unknown.[3]
Legality
D-lysergic acid α-hydroxyethylamide is unscheduled and uncontrolled in the United States, but possession and sales of it for human consumption could potentially be prosecuted under the Federal Analog Act because of its structural similarities to LSD.
See also
- Ergoline
- Lysergic acid
- LSA
- LSD
- Ergot
- Hawaiian baby woodrose (Argyreia nervosa)
- Ololiuhqui (Rivea corymbosa)
- Tlitliltzin (Ipomoea violacea)
References
- "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- Glasser A (January 1961). "Some pharmacological actions of D-lysergic acid methyl carbinolamide". Nature. 189 (4761): 313–4. Bibcode:1961Natur.189..313G. doi:10.1038/189313a0. PMID 13705953. S2CID 4260358.
- Hofmann A (1971). "Teonanácatl and Ololiuqui, two ancient magic drugs of Mexico". Bulletin on Narcotics. 1: 3–14.
External links
- Ergot - A Rich Source of Pharmacologically Active Substances by Albert Hofmann