Epicriptine
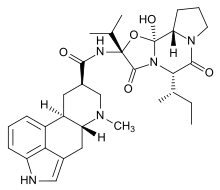
Epicriptine or beta-dihydroergocryptine is a dopamine agonist of the ergoline class. It constitutes one third of the mixture known as dihydroergocryptine, the other two thirds consisting of alpha-dihydroergocryptine. The alpha differs from the beta form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid isoleucine is replaced by leucine.[1]
 | |
| Clinical data | |
|---|---|
| Other names | beta-Dihydroergocryptine |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.039.157 |
| Chemical and physical data | |
| Formula | C32H43N5O5 |
| Molar mass | 577.726 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Steinhilber D, Schubert-Zsilavecz M, Roth HJ (2005). Medizinische Chemie (in German). Stuttgart: Deutscher Apotheker Verlag. p. 142. ISBN 3-7692-3483-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.