Benperidol
Benperidol, sold under the trade name Anquil[1] among others, is a typical antipsychotic primarily used to treat hypersexuality syndromes[2] and can be used to treat schizophrenia.[3] It is a highly potent butyrophenone derivative and is the most potent neuroleptic on the European market, with chlorpromazine equivalency as high as 75 to 100 (about 150 to 200% the potency, per dose, of haloperidol).[4] It is sometimes prescribed to sex offenders as a condition of their parole, as an alternative to anti-androgen drugs such as cyproterone acetate.[5]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anquil, Frenactil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 8 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.521 |
| Chemical and physical data | |
| Formula | C22H24FN3O2 |
| Molar mass | 381.451 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Benperidol was discovered by Janssen Pharmaceutica in 1961 and has been marketed since 1966. It is mainly used in Germany, but it is also available in Belgium, Greece, Italy, the Netherlands, and the United Kingdom.[6]
Pharmacology
Pharmacodynamics
Benperidol is a strong dopamine receptor antagonist (D2 (Ki 0.027 nM) and D4 (Ki 0.066 nM))[7] with weaker serotonin receptor antagonism (5-HT2A (Ki 3.75 nM))[7]. In high doses, it has antihistaminergic and alpha-adrenergic properties. It possesses minimal anticholinergic properties.[8]
| Site | Ki (nM) | Action | Ref |
|---|---|---|---|
| 5-HT2A | 3.75 | Antagonist | [7] |
| D1 | 4,100 | Antagonist | [7] |
| D2 | 0.027 | Antagonist | [7] |
| D4 | 0.06 | Antagonist | [7] |
Pharmacokinetics
Benperidol is absorbed well and undergoes extensive first pass metabolism. One percent of benperidol is excreted in urine. The half-life of benperidol is 8 hours.[8]
Synthesis
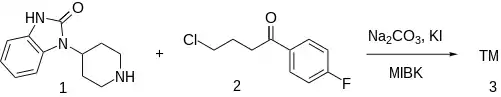
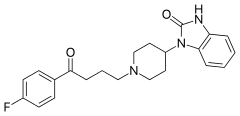
4-(2-Keto-1-benzimidazolinyl)piperidine [20662-53-7] (1) is alkylated with 4-Chloro-4'-Fluorobutyrophenone [3874-54-2] (2).
See also
- Timiperone has a similar chemical structure with a thiourea group instead of a urea group.
- Pimozide & Bezitramide (& Oxiperomide & Neflumozide) are also made from 4-(1-Benzimidazolinone)piperidine precursor
- Droperidol is similar, but has a tetrahydropyridine ring.
References
- Council A, Kuenssberg V (1974-02-01). "Benperidol - a drug for sexual offenders?". Drug and Therapeutics Bulletin. BMJ Publishing Group Ltd. 12 (3): 12. doi:10.1136/dtb.12.3.12. PMID 4457302. S2CID 44581451.
- British National Formulary (49th), British Medical Association 2005 p 183
- Bobon J, Collard J, Lecoq R (October 1963). "[Benperidol and promazine: a "double blind" comparative study in mental geriatrics]". Acta Neurologica et Psychiatrica Belgica (in French). 63: 839–43. PMID 14092279.
- Möller HJ, Müller WE, Bandelow (2001). Neuroleptika: pharmakologische Grundlagen, klinisches Wissen und therapeutisches Vorgehen; mit 136 Tabellen (in German). Wiss. Verlag-Ges. ISBN 3-8047-1773-X.
- Murray MA, Bancroft JH, Anderson DC, Tennent TG, Carr PJ (November 1975). "Endocrine changes in male sexual deviants after treatment with anti-androgens, oestrogens or tranquillizers". The Journal of Endocrinology. 67 (2): 179–88. doi:10.1677/joe.0.0670179. PMID 1107462.
- "NCATS Inxight Drugs — BENPERIDOL". Retrieved 13 March 2022.
- Li P, Snyder GL, Vanover KE (December 2016). "Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future". Current Topics in Medicinal Chemistry. 16 (29): 3385–3403. doi:10.2174/1568026616666160608084834. PMC 5112764. PMID 27291902.
- Leucht S, Hartung B (April 2005). "Benperidol for schizophrenia". The Cochrane Database of Systematic Reviews. 2005 (2): CD003083. doi:10.1002/14651858.CD003083.pub2. PMC 7017029. PMID 15846648.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 11 March 2022.
- BE 626307 (1963 to Janssen), C.A. 60, 10690c (1964), corresp. to GB 989755, "1-(1-aroylpropyl-4-piperidyl)-2-benzimidazolinones and related compounds", published 1965-04-22, assigned to N.V. Research Laboratorium Dr. C. Janssen.