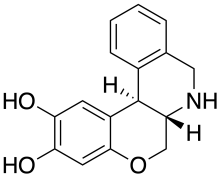
Doxanthrine
Doxanthrine is a synthetic compound which is a potent and selective full agonist for the dopamine D1 receptor.[1][2] Doxanthrine has been shown to be orally active in producing contralateral rotation in the 6-hydroxydopamine rat model of Parkinson's disease.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C16H15NO3 |
| Molar mass | 269.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- Cueva JP, Giorgioni G, Grubbs RA, Chemel BR, Watts VJ, Nichols DE (November 2006). "trans-2,3-dihydroxy-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline: synthesis, resolution, and preliminary pharmacological characterization of a new dopamine D1 receptor full agonist". Journal of Medicinal Chemistry. 49 (23): 6848–57. doi:10.1021/jm0604979. PMID 17154515.
- Przybyla JA, Cueva JP, Chemel BR, Hsu KJ, Riese DJ, McCorvy JD, Chester JA, Nichols DE, Watts VJ (February 2009). "Comparison of the enantiomers of (±)-doxanthrine, a high efficacy full dopamine D1 receptor agonist, and a reversal of enantioselectivity at D1 versus alpha2C adrenergic receptors". European Neuropsychopharmacology. 19 (2): 138–46. doi:10.1016/j.euroneuro.2008.10.002. PMC 2636714. PMID 19028082.
- McCorvey JD, Watts VJ, Nichols DE (July 2012). "Comparison of the D1 dopamine full agonists, dihydrexidine and doxanthrine, in the 6-OHDA rat model of Parkinson's disease". Psychopharmacology. 222 (1): 81–87. doi:10.1007/s00213-011-2625-5. PMID 22222862. S2CID 7641172.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.