Spiperone
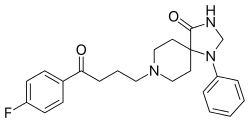
Spiperone (Spiroperidol; brand name: Spiropitan (JP)) is a typical antipsychotic and research chemical belonging to the butyrophenone chemical class.[1] It is licensed for clinical use in Japan as a treatment for schizophrenia.[2] Additionally, spiperone was identified by compound screening to be an activator of Ca2+ activated Cl− channels (CaCCs), thus a potential target for therapy of cystic fibrosis.[3]
| Receptor | Ki (nM)[4] | Notes |
|---|---|---|
| 5-HT1A | 17.3 | |
| 5-HT1B | 995 | |
| 5-HT1D | 2397 | |
| 5-HT1E | 5051 | |
| 5-HT1F | 3.98 | |
| 5-HT2A | 1.17 | |
| 5-HT2B | 1114.2 | |
| 5-HT2C | 922.9 | |
| 5-HT3 | >10000 | No data available from cloned human receptors. Data comes from rat cortex receptors and other sources. |
| 5-HT5A | 2512 | Cloned mouse receptor. |
| 5-HT6 | 1590 | Cloned rat receptor. |
| 5-HT7 | 109.8 | |
| α1A | 20.4 | |
| α1B | 3.09 | |
| α1D | 8.32 | |
| D1 | 398.5 | |
| D2 | 0.16 | |
| D3 | 0.34 | |
| D4 | 1.39 | |
| D5 | 4500 | |
| H1 | 272 | |
| σ | 353 |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.931 |
| Chemical and physical data | |
| Formula | C23H26FN3O2 |
| Molar mass | 395.478 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
N-Methylspiperone (NMSP) is a derivate of spiperone that is used to study the dopamine and serotonin neurotransmitter system. Labeled with the radioisotope carbon-11, it can be used for positron emission tomography.[5]
References
- Zheng LT, Hwang J, Ock J, Lee MG, Lee WH, Suk K (September 2008). "The antipsychotic spiperone attenuates inflammatory response in cultured microglia via the reduction of proinflammatory cytokine expression and nitric oxide production". J. Neurochem. 107 (5): 1225–35. doi:10.1111/j.1471-4159.2008.05675.x. PMID 18786164.
- Mirtazapine. Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 12 September 2011. Retrieved 4 November 2013.
- Liang L, Macdonald KD, Schwiebert EM, Zeitlin PL, Guggino WB (October 2008). "Spiperone, Identified through Compound Screening, Activates Calcium Dependent Chloride Secretion in the Airway". Am J Physiol Cell Physiol. 296 (1): C131–41. doi:10.1152/ajpcell.00346.2008. PMC 4116347. PMID 18987251.
- Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 4 November 2013.
- Bengt Andree; et al. (August 1998). "Positron Emission Tomographic Analysis of Dose-dependent MDL-100,907 Binding to 5-Hydroxtryptamine-2A Receptors in the Human Brain". Journal of Clinical Psychopharmacology. 18 (4): 313–323.
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.