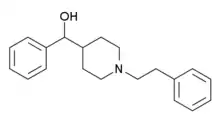
Glemanserin
Glemanserin (INN) (developmental code name MDL-11,939) is a drug which acts as a potent and selective 5-HT2A receptor antagonist.[1] The first truly selective 5-HT2A ligand to be discovered, glemanserin resulted in the development of the widely used and even more potent and selective 5-HT2A receptor antagonist volinanserin (MDL-100,907), which is a fluorinated analogue.[2] Though it was largely superseded in scientific research by volinanserin, glemanserin was investigated clinically for the treatment of generalized anxiety disorder.[3] However, it was ultimately found to be ineffective and was not marketed.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H25NO |
| Molar mass | 295.426 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
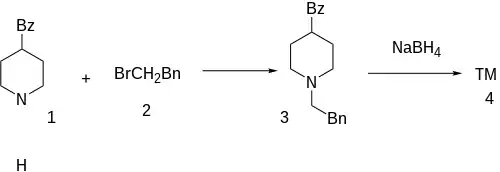
Synthesis
The alkylation of 4-benzoylpiperidine [37586-22-4] (1) with 2-Phenylethylbromide [103-63-9] (2) gives (1-Phenethyl-piperidin-4-yl)-phenyl-methanone, CID:10379653 (3). The reduction of the carbonyl ketone to an alcohol with sopdium borohydride completed the synthesis of Glemanserin (4).
References
- Mark W. Dudley; Norbert L. Wiech; Francis P. Miller; et al. (1988). "Pharmacological effects of MDL 11,939: A selective, centrally acting antagonist of 5-HT2 receptors". Drug Development Research. 13 (1): 29–43. doi:10.1002/ddr.430130104. S2CID 85075328.
- Berend Olivier (10 July 1997). Serotonin Receptors and Their Ligands. Elsevier. p. 167. ISBN 978-0-444-82041-9. Retrieved 6 May 2012.
- Sramek JJ, Robinson RE, Suri A, Cutler NR (February 1995). "Efficacy trial of the 5-HT2 antagonist MDL 11,939 in patients with generalized anxiety disorder". Journal of Clinical Psychopharmacology. 15 (1): 20–2. doi:10.1097/00004714-199502000-00004. PMID 7714223.
- Stephen M. Sorensen, EP 0317933 (1989 to Merrell Dow Pharmaceuticals Inc.).
- Francis P. Miller, Albert A. Carr, EP 0319962 (1989 to Merrell Dow Pharmaceuticals Inc.).
- Paul J. Schechter, John M. Orwin, Christian Karl Hinze, EP 0325063 (1994 to Merrell Dow Pharmaceuticals Inc.).
- Albert A. Carr, Norbert L. Wiech, EP 0208235 (1990 to Merrell Dow Pharmaceuticals Inc.).
- Albert A. Carr, Norbert L. Wiech, U.S. Patent 4,783,471 (1988 to Merrell Dow Pharmaceuticals Inc.).