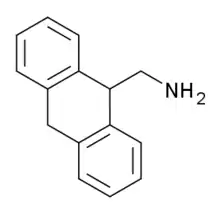
9-Aminomethyl-9,10-dihydroanthracene
AMDA (9-Aminomethyl-9,10-dihydroanthracene) is an organic compound which acts as a potent and selective antagonist for the 5-HT2A receptor.[1] It has been used to help study the shape of the 5-HT2A protein,[2] and develop a large family of related derivatives with even higher potency and selectivity.[3][4][5][6][7]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H15N |
| Molar mass | 209.292 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- Westkaemper RB, Runyon SP, Bondarev ML, Savage JE, Roth BL, Glennon RA (September 1999). "9-(Aminomethyl)-9,10-dihydroanthracene is a novel and unlikely 5-HT2A receptor antagonist". European Journal of Pharmacology. 380 (1): R5-7. doi:10.1016/S0014-2999(99)00525-7. PMID 10513561.
- Runyon SP, Peddi S, Savage JE, Roth BL, Glennon RA, Westkaemper RB (April 2002). "Geometry-affinity relationships of the selective serotonin receptor ligand 9-(aminomethyl)-9,10-dihydroanthracene". Journal of Medicinal Chemistry. 45 (8): 1656–64. doi:10.1021/jm010354g. PMID 11931619.
- Peddi S, Roth BL, Glennon RA, Westkaemper RB (December 2003). "Spiro[9,10-dihydroanthracene]-9,3'-pyrrolidine-a structurally unique tetracyclic 5-HT2A receptor antagonist". European Journal of Pharmacology. 482 (1–3): 335–7. doi:10.1016/j.ejphar.2003.09.059. PMID 14660041.
- Peddi S, Roth BL, Glennon RA, Westkaemper RB (May 2004). "Structural determinants for high 5-HT(2A) receptor affinity of spiro[9,10-dihydroanthracene]-9,3(')-pyrrolidine (SpAMDA)". Bioorganic & Medicinal Chemistry Letters. 14 (9): 2279–83. doi:10.1016/j.bmcl.2004.02.014. PMID 15081025.
- Dewkar GK, Peddi S, Mosier PD, Roth BL, Westkaemper RB (October 2008). "Methoxy-substituted 9-aminomethyl-9,10-dihydroanthracene (AMDA) derivatives exhibit differential binding affinities at the 5-HT(2A) receptor". Bioorganic & Medicinal Chemistry Letters. 18 (19): 5268–71. doi:10.1016/j.bmcl.2008.08.059. PMC 3082371. PMID 18774714.
- Runyon SP, Mosier PD, Roth BL, Glennon RA, Westkaemper RB (November 2008). "Potential modes of interaction of 9-aminomethyl-9,10-dihydroanthracene (AMDA) derivatives with the 5-HT2A receptor: a ligand structure-affinity relationship, receptor mutagenesis and receptor modeling investigation". Journal of Medicinal Chemistry. 51 (21): 6808–28. doi:10.1021/jm800771x. PMC 3088499. PMID 18847250.
- Shah JR, Mosier PD, Peddi S, Roth BL, Westkaemper RB (February 2010). "9-Aminomethyl-9,10-dihydroanthracene (AMDA) analogs as structural probes for steric tolerance in 5-HT2A and H1 receptor binding sites". Bioorganic & Medicinal Chemistry Letters. 20 (3): 935–8. doi:10.1016/j.bmcl.2009.12.064. PMC 3252747. PMID 20045641.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.