3,4-Methylenedioxyamphetamine
3,4-Methylenedioxyamphetamine (also known as MDA and sass) is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In terms of pharmacology, MDA acts most importantly as a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, sublingual, insufflation, intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP extensively involved) |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.706 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
MDA is rarely sought after as a recreational drug compared to other drugs in the amphetamine family; however, it remains an important and widely used drug due to it being a primary metabolite,[1] the product of hepatic N-dealkylation,[2] of MDMA (ecstasy). In addition, it is common to find MDA as an adulterant of illicitly produced MDMA.[3][4]
Uses
Medical
MDA currently has no accepted medical use.
Adverse effects
MDA produces serotonergic neurotoxic effects,[7][8] thought to be activated by initial metabolism of MDA.[2] In addition, MDA activates a response of the neuroglia, though this subsides after use.[7]
Overdose
Symptoms of acute toxicity may include agitation, sweating, increased blood pressure and heart rate, dramatic increase in body temperature, convulsions, and death. Death is usually caused by cardiac effects and subsequent hemorrhaging in the brain (stroke).[9]
Pharmacology
Pharmacodynamics
MDA is a substrate of the serotonin, norepinephrine, dopamine, and vesicular monoamine transporters, as well as a TAAR1 agonist,[10] and for these reasons acts as a reuptake inhibitor and releasing agent of serotonin, norepinephrine, and dopamine (that is, it is an SNDRA).[11] It is also an agonist of the serotonin 5-HT2A,[12] 5-HT2B,[13] and 5-HT2C receptors[14] and shows affinity for the α2A-, α2B-, and α2C-adrenergic receptors and serotonin 5-HT1A and 5-HT7 receptors.[15]
The (S)-optical isomer of MDA is more potent than the (R)-optical isomer as a psychostimulant, possessing greater affinity for the three monoamine transporters.
In terms of the subjective and behavioral effects of MDA, it is thought that serotonin release is required for its empathogen-entactogen effects, release of dopamine and norepinephrine is responsible for its psychostimulant effects, dopamine release is necessary for its euphoriant (rewarding and addictive) effects, and direct agonism of the serotonin 5-HT2A receptor is causative of its psychedelic effects.
Pharmacokinetics
The duration of the drug has been reported as about 6 to 8 hours.[6]
Chemistry
MDA is a substituted methylenedioxylated phenethylamine and amphetamine derivative. In relation to other phenethylamines and amphetamines, it is the 3,4-methylenedioxy, α-methyl derivative of β-phenylethylamine, the 3,4-methylenedioxy derivative of amphetamine, and the N-desmethyl derivative of MDMA.
Synonyms
In addition to 3,4-methylenedioxyamphetamine, MDA is also known by other chemical synonyms such as the following:
- α-Methyl-3,4-methylenedioxy-β-phenylethylamine
- 1-(3,4-Methylenedioxyphenyl)-2-propanamine
- 1-(1,3-Benzodioxol-5-yl)-2-propanamine
Synthesis
MDA is typically synthesized from essential oils such as safrole or piperonal. Common approaches from these precursors include:
- Reaction of safrole's alkene functional group with a halogen containing mineral acid followed by amine alkylation.[16][17]
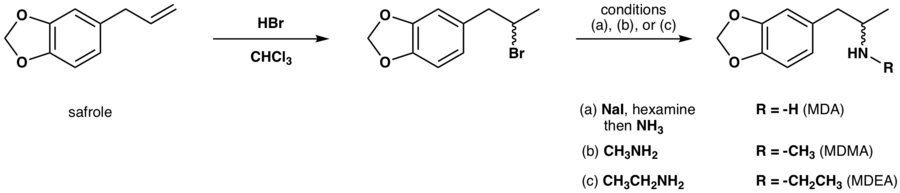
- Wacker oxidation of safrole to yield 3,4-methylenedioxyphenylpropan-2-one (MDP2P) followed by reductive amination[17][18] or via reduction of its oxime.[19]
- Henry reaction of piperonal with nitroethane followed by nitro compound reduction.[17][20][21][22][23]
- Darzens reaction on heliotropin was also done by J. Elks, et al.[24] This gives MDP2P, which was then subjected to a Leuckart reaction.
Detection in body fluids
MDA may be quantitated in blood, plasma or urine to monitor for use, confirm a diagnosis of poisoning or assist in the forensic investigation of a traffic or other criminal violation or a sudden death. Some drug abuse screening programs rely on hair, saliva, or sweat as specimens. Most commercial amphetamine immunoassay screening tests cross-react significantly with MDA and major metabolites of MDMA, but chromatographic techniques can easily distinguish and separately measure each of these substances. The concentrations of MDA in the blood or urine of a person who has taken only MDMA are, in general, less than 10% those of the parent drug.[25][26][27]
Derivatives
MDA constitutes part of the core structure of the β-adrenergic receptor agonist protokylol.
History
MDA was first synthesized by C. Mannich and W. Jacobsohn in 1910.[19] It was first ingested in July 1930 by Gordon Alles who later licensed the drug to Smith, Kline & French.[28] MDA was first used in animal tests in 1939, and human trials began in 1941 in the exploration of possible therapies for Parkinson's disease. From 1949 to 1957, more than 500 human subjects were given MDA in an investigation of its potential use as an antidepressant and/or anorectic by Smith, Kline & French. The United States Army also experimented with the drug, code named EA-1298, while working to develop a truth drug or incapacitating agent. Harold Blauer[29] died in January 1953 after being intravenously injected, without his knowledge or consent, with 450 mg of the drug as part of Project MKUltra. MDA was patented as an ataractic by Smith, Kline & French in 1960, and as an anorectic under the trade name "Amphedoxamine" in 1961. MDA began to appear on the recreational drug scene around 1963 to 1964. It was then inexpensive and readily available as a research chemical from several scientific supply houses. Several researchers, including Claudio Naranjo and Richard Yensen, have explored MDA in the field of psychotherapy.[30][31]
Society and culture

Name
When MDA was under development as a potential pharmaceutical drug, it was given the international nonproprietary name (INN) of tenamfetamine.
Australia
MDA is schedule 9 prohibited substance under the Poisons Standards.[32] A schedule 9 substance is listed as a "Substances which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities."[32]
United States
MDA is a Schedule I controlled substance in the US.
Research
In 2010, the ability of MDA to invoke mystical experiences and alter vision in healthy volunteers was studied. The study concluded that MDA is a "potential tool to investigate mystical experiences and visual perception".[6]
References
- Crean, R. D.; Davis, S. A.; Von Huben, S. N.; Lay, C. C.; Katner, S. N.; Taffe, M. A. (13 October 2006). "Effects of (±)3,4-methylenedioxymethamphetamine, (±)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques". Neuroscience. 142 (2): 515–525. doi:10.1016/j.neuroscience.2006.06.033. PMC 1853374. PMID 16876329.
- de la Torre, R; Farre, M; Roset, Pn; Pizzaro, N; Abanades, S; Segura, M; Segura, M; Camí, J (2004). "Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition". Therapeutic Drug Monitoring. 26 (2): 137–144. doi:10.1097/00007691-200404000-00009. PMID 15228154.
- EcstasyData.org. "EcstasyData.org: Test Result Statistics: Substances by Year". www.ecstasydata.org. Retrieved 27 June 2017.
- "Trans European Drug Information". idpc.net. Retrieved 27 June 2017.
- Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE (1993). "Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine". Journal of Medicinal Chemistry. 36 (23): 3700–3706. doi:10.1021/jm00075a027. PMID 8246240.
- Baggott, MJ; Siegrist, JD; Galloway, GP; Robertson, LC; Coyle, JR; Mendelson, JE (2010). "Investigating the Mechanisms of Hallucinogen-Induced Visions Using 3,4-Methylenedioxeamphetamine (MDA): A Randomized Controlled Trial in Humans". PLOS ONE. 5 (12): e14074. Bibcode:2010PLoSO...514074B. doi:10.1371/journal.pone.0014074. PMC 2996283. PMID 21152030.
- Herndon, Joseph M.; Cholanians, Aram B.; Lau, Serrine S.; Monks, Terrence J. (March 2014). "Glial Cell Response to 3,4-(±)-Methylenedioxymethamphetamine and Its Metabolites". Toxicological Sciences. 138 (1): 130–138. doi:10.1093/toxsci/kft275. ISSN 1096-6080. PMC 3930364. PMID 24299738.
- Kalant, Harold (2 October 2001). "The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs". CMAJ: Canadian Medical Association Journal. 165 (7): 917–928. ISSN 0820-3946. PMC 81503. PMID 11599334.
- Diaz, Jaime (1996). How Drugs Influence Behavior. Englewood Cliffs: Prentice Hall.
- Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–8. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem. 6 (17): 1845–59. doi:10.2174/156802606778249766. PMID 17017961.
- Giuseppe Di Giovanni; Vincenzo Di Matteo; Ennio Esposito (2008). Serotonin–dopamine Interaction: Experimental Evidence and Therapeutic Relevance. Elsevier. pp. 294–. ISBN 978-0-444-53235-0.
- Rothman, Richard B; Baumann, Michael H (2009). "Serotonergic drugs and valvular heart disease". Expert Opinion on Drug Safety. 8 (3): 317–329. doi:10.1517/14740330902931524. ISSN 1474-0338. PMC 2695569. PMID 19505264.
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA (1994). "Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors". Neurosci. Lett. 177 (1–2): 111–5. doi:10.1016/0304-3940(94)90057-4. PMID 7824160. S2CID 41352480.
- Manzoni, Olivier Jacques; Ray, Thomas S. (2010). "Psychedelics and the Human Receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. ISSN 1932-6203. PMC 2814854. PMID 20126400.
- Muszynski, I.E. (1961). "Production of some amphetamine derivatives". Acta Poloniae Pharmaceutica. 18: 471–478. PMID 14477621.
- Shulgin, Alexander; Manning, Tania; Daley, Paul (2011). The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds (1st ed.). Berkeley, CA: Transform Press. p. 165. ISBN 9780963009630.
- Noggle, FT Jr; DeRuiter, J.; Long, MJ. (1986). "Spectrophotometric and liquid chromatographic identification of 3,4-methylenedioxyphenylisopropylamine and its N-methyl and N-ethyl homologs". Journal of the Association of Official Analytical Chemists. 69 (4): 681–686. PMID 2875058.
- Mannich, C.; Jacobsohn, W.; Mannich, Hr. C. (1910). "Über Oxyphenyl-alkylamine und Dioxyphenyl-alkylamine". Berichte der Deutschen Chemischen Gesellschaft. 41 (1): 189–197. doi:10.1002/cber.19100430126.
- Ho, Beng-Thong; McIsaac, William M.; An, Rong; Tansey, L. Wayne; Walker, Kenneth E.; Englert Jr., Leo F.; Noel, Michael B. (1970). "Analogs of a-methylphenethylamine". Journal of Medicinal Chemistry. 13 (1): 26–30. doi:10.1021/jm00295a007. PMID 5412110.
- Butterick, John R.; Unrau, A. M. (1974). "Reduction of β-nitrostyrene with sodium bis-(2-methoxyethoxy)-aluminium dihydride. A convenient route to substituted phenylisopropylamines". Journal of the Chemical Society, Chemical Communications. 8 (8): 307–308. doi:10.1039/C39740000307.
- Toshitaka, Ohshita; Hiroaka, Ando (1992). "Synthesis of Phenethylamine Derivatives as Hallucinogen". Japanese Journal of Toxicology and Environmental Health. 38 (6): 571–580. doi:10.1248/jhs1956.38.571. Retrieved 20 June 2014.
- Shulgin, Alexander & Shulgin, Ann (1991). PiHKAL: A Chemical Love Story. Lafayette, CA: Transform Press. ISBN 9780963009609.
- Elks, J.; Hey, D. H. (1943). "7. β-3 : 4-Methylenedioxyphenylisopropylamine". J. Chem. Soc.: 15–16. doi:10.1039/JR9430000015. ISSN 0368-1769.
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methyl
enedioxy methamphetamine after controlled oral administration to young adults. Ther. Drug Monit. 30: 320–332, 2008. - Barnes AJ, De Martinis BS, Gorelick DA, Goodwin RS, Kolbrich EA, Huestis MA (2009). "Disposition of MDMA and metabolites in human sweat following controlled MDMA administration". Clinical Chemistry. 55 (3): 454–62. doi:10.1373/clinchem.2008.117093. PMC 2669283. PMID 19168553.
- R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, California, 2011, pp. 1078–1080.
- "The First MDA trip and the measurement of 'mystical experience' after MDA, LSD, and Psilocybin". Psychedelic research. 18 July 2008. Archived from the original on 13 July 2012.
- The History Channel documented details of his death here https://www.youtube.com/watch?v=ySw-0uY4CUA See minute 2:38 onward.
- Naranjo, C.; Shulgin, A. T.; Sargent, T. (1967). "Evaluation of 3, 4-methylenedioxeamphetamine (MDA) as an adjunct to psychotherapy". Pharmacology. 17 (4): 359–364. doi:10.1159/000137100. PMID 5631047.
- Yensen, R.; Di Leo, F. B.; Rhead, J. C.; Richards, W. A.; Soskin, R. A.; Turek, B.; Kurland, A. A. (1976). "MDA-assisted psychotherapy with neurotic outpatients: a pilot study". The Journal of Nervous and Mental Disease. 163 (4): 233–245. doi:10.1097/00005053-197610000-00002. PMID 972325. S2CID 41155810.
- Poisons Standard (October 2015) comlaw.gov.au