Trifluoromescaline
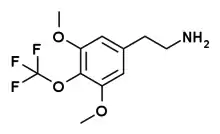
Trifluoromescaline (TF-M) is a derivative of the phenethylamine hallucinogen mescaline, which has a trifluoromethoxy group replacing the central methoxy group of mescaline. Synthesis of this compound was first reported by Daniel Trachsel in 2011, alongside many other related compounds.[1][2] Trifluoromescaline was found to be one of the most potent compounds in the series, with a reported dosage of 15-40 mg (and 60 mg being described as a "strong overdose"), and a slow onset of action and long duration of effects, lasting 14-24 hours or more.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C11H14F3NO3 |
| Molar mass | 265.232 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- 2C-TFM
- 2C-TFE
- 2C-T-28
- 3C-DFE
- 4-TM
- Allylescaline
- 2-Bromomescaline
References
- Trachsel D (2002). "Synthese von neuen (Phenylalkyl)aminen zur Untersuchung von Struktur-Aktivitätsbeziehungen, Mitteilung 1, Mescalin Derivate". Helvetica Chimica Acta. 85 (9): 3019–3026. doi:10.1002/1522-2675(200209)85:9<3019::AID-HLCA3019>3.0.CO;2-4.
- Trachsel D (2012). "Fluorine in psychedelic phenethylamines". Drug Testing and Analysis. 4 (7–8): 577–90. doi:10.1002/dta.413. PMID 22374819.
- Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine Von der Struktur zur Funktion. Nachtschatten Verlag AG. pp. 704–723. ISBN 978-3-03788-700-4.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.