Baeocystin
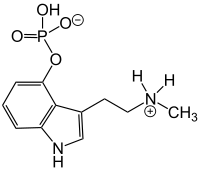
Baeocystin is a zwitterionic alkaloid and analog of psilocybin. It is found as a minor compound in most psilocybin mushrooms together with psilocybin, norbaeocystin, and psilocin. Baeocystin is an N-demethylated derivative of psilocybin, and a phosphorylated derivative of 4-HO-NMT (4-hydroxy-N-methyltryptamine). The structures at right illustrate baeocystin in its zwitterionic form.
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H15N2O4P |
| Molar mass | 270.225 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Baeocystin was first isolated from the mushroom Psilocybe baeocystis,[1] and later from P. semilanceata,[2] Panaeolus renenosus, Panaeolus subbalteatus, and Copelandia chlorocystis.[3] It was first synthesized by Troxler et al. in 1959.[4]
Little information exists with regard to human pharmacology, but in the book Magic Mushrooms Around the World, author Jochen Gartz reports being aware of a study in which "10 mg of baeocystin were found to be about as psychoactive as a similar amount of psilocybin."[5] Gartz also reported in a research paper that a self-administered assay of 4 mg of baeocystin caused "a gentle hallucinogenic experience".[6]
While Gartz describes experiencing a "gentle hallucinogenic experience" from baeocystin, this could not be replicated in a mouse model in a 2019 study which found no evidence that baeocystin produces any hallucinogenic effects.[7] Researchers compared psilocybin which is a known hallucinogen to baeocystin by using the mouse head-twitch response. Upon comparison, baeocystin was indistinguishable from saline solution, indicating "...baeocystin alone would likely not induce hallucinogenic effects in vivo". This does contrast however with the human experiences mentioned above, although high quality data is scarce.
See also
References
- Leung AY, Paul AG (1968). "Baeocystin and norbaeocystin: New analogs of psilocybin from Psilocybe baeocystis". Journal of Pharmaceutical Sciences. 57 (10): 1667–71. doi:10.1002/jps.2600571007. PMID 5684732.
- Repke DB, Leslie DT, Guzman G (1977). "Baeocystin in Psilocybe, Conocybe and Panaeolus". Lloydia. 40 (6): 566–78. PMID 600026.
- Brossi A. (1988). The Alkaloids: Chemistry and Pharmacology V32: Chemistry and Pharmacology. Academic Press. p. 46. ISBN 978-0-08-086556-0.
- Troxler, F.; Seeman, F.; Hofmann, A. (1959). "Abwandlungsprodukte von Psilocybin und Psilocin. 2. Mitteilung über synthetische Indolverbindungen". Helvetica Chimica Acta (in German). 42 (6): 2073–2103. doi:10.1002/hlca.19590420638.
- Gartz J. (1997). Magic Mushrooms Around the World. Los Angeles, California: LIS Publications. p. 27. ISBN 978-0-9653399-0-2.
- Gartz J. (1991). "Further Investigations on Psychoactive Mushrooms of the Genera Psylocibe, Gymnopilus and Conocybe" (PDF). Ann. Mus. Civ. Rovereto. 7: 265–74.
- Sherwood, Alexander (2020). "Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin". Journal of Natural Products. 83 (2): 461–467. doi:10.1021/acs.jnatprod.9b01061. PMID 32077284.