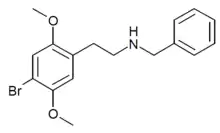
N-Benzyl-2C-B
N-Benzyl-2C-B (25B-NB, NB-2C-B) is a recreational designer drug from the 25-NB subgroup of the substituted phenethylamine family, with psychedelic effects. It has a binding affinity (Ki) of 16 nM at the serotonin receptor 5-HT2A and 90 nM at 5-HT2C and reportedly has a potency in between that of 2C-B and NBOMe-2C-B.[1][2]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H20BrNO2 |
| Molar mass | 350.256 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Glennon RA, Dukat M, el-Bermawy M, Law H, De los Angeles J, Teitler M, King A, Herrick-Davis K (June 1994). "Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines". Journal of Medicinal Chemistry. 37 (13): 1929–35. doi:10.1021/jm00039a004. PMID 8027974.
- Halberstadt AL (2017). "Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens". In Baumann M, Glennon R, Wiley J (eds.). Neuropharmacology of New Psychoactive Substances (NPS). Current Topics in Behavioral Neurosciences. Vol. 32. Cham: Springer. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3. PMID 28097528.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.