Methamnetamine
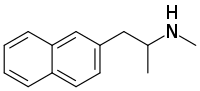
Methamnetamine (also known as methylnaphetamine, MNA, MNT and PAL-1046) is a triple monoamine releasing agent and N-methyl analog of the non-neurotoxic experimental drug naphthylaminopropane and the naphthalene analog of methamphetamine.[1][2][3] It has been sold online as a designer drug.[4][5]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C14H17N |
| Molar mass | 199.297 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It acts as a releasing agent of serotonin, norepinephrine, and dopamine, with EC50 values of 13 nM, 34 nM, and 10 nM, respectively.[1]
Legal status
Methamnetamine is illegal in Japan.[6]
References
- Rothman RB, Partilla JS, Baumann MH, Lightfoot-Siordia C, Blough BE (April 2012). "Studies of the biogenic amine transporters. 14. Identification of low-efficacy "partial" substrates for the biogenic amine transporters". The Journal of Pharmacology and Experimental Therapeutics. 341 (1): 251–62. doi:10.1124/jpet.111.188946. PMC 3364510. PMID 22271821.
- Youn, Dong-Hyun; Kim, Jin Mook; Hong, Young-ki; Park, Seo-In; Lee, Jin-Moo; Kim, Young-Hoon; Park, Chang Won; Kang, Mi Sun (April 2021). "Assessment of the abuse potential of methamnetamine in rodents: a behavioral pharmacology study". Psychopharmacology. 238 (8): 2155–2165. doi:10.1007/s00213-021-05840-9. ISSN 1432-2072. PMID 33811503. S2CID 232773019.
- Hong, Young-ki; Kim, Young-Hoon; Lee, Jin-Moo; Yoo, Hye Hyun; Choi, Sun-Ok; Kang, Mi Sun (April 2021). "Characterization of in vitro phase I metabolites of methamnetamine in human liver microsomes by liquid chromatography-quadrupole time-of-flight mass spectrometry". International Journal of Legal Medicine. 135 (4): 1471–1476. doi:10.1007/s00414-021-02594-z. ISSN 1437-1596. PMID 33928430. S2CID 233451101.
- "Methamnetamine". WEDINOS.
- "Methamnetamine". New Synthetic Drugs Database.
- "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 8 October 2015.
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.