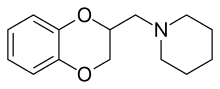
Piperoxan
Piperoxan, also known as benodaine, was the first antihistamine to be discovered.[1][2] This compound, derived from benzodioxan, was prepared in the early 1930s by Daniel Bovet and Ernest Fourneau at the Pasteur Institute in France.[1][2] Formerly investigated by Fourneau as an α-adrenergic-blocking agent, they demonstrated that it also antagonized histamine-induced bronchospasm in guinea pigs, and published their findings in 1933.[1][2][3] Bovet went on to win the 1957 Nobel Prize in Physiology or Medicine for his contribution.[4] One of Bovet and Fourneau's students, Anne-Marie Staub, published the first structure–activity relationship (SAR) study of antihistamines in 1939.[1] Piperoxan and analogues themselves were not clinically useful due to the production of toxic effects in humans and were followed by phenbenzamine (Antergan) in the early 1940s, which was the first antihistamine to be marketed for medical use.[5]
 | |
| Clinical data | |
|---|---|
| Other names | Benodaine |
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.311 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
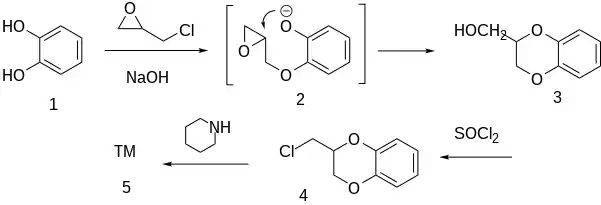
Synthesis
Condensation of catechol [120-80-9] (1) with epichlorohydrin in the presence of an aqueous base can be visualized as proceeding initially with the epoxide (2) Opening of the oxirane ring by the phenoxide anion then leads to 2-hydroxymethyl-1,4-benzodioxane [3663-82-9] (3). Halogenation with thionyl chloride gives 2-chloromethyl-1,4-benzodioxane [2164-33-2] (4). Displacement of the leaving group by piperidine completed the synthesis of piperoxan (5).
References
- Scriabine A, Landau R, Achilladelis B (1999). Pharmaceutical innovation: revolutionizing human health. Philadelphia: Chemical Heritage Press. ISBN 0-941901-21-1.
- Williams DH, Lemke TL, Foye WO (2008). Foye's principles of medicinal chemistry. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-6879-5.
- Fourneau E, Bovet D (1933). "Recherches sur l'action sympathicolytique d'un nouveau dérivé du dioxane". Archives Internationales de Pharmacodynamie et de Thérapie. 46: 178–91. ISSN 0003-9780.
- "Daniel Bovet - Biography". NobelPrize.org.
- Ralph Landau; Basil Achilladelis; Alexander Scriabine (1999). Pharmaceutical Innovation: Revolutionizing Human Health. Chemical Heritage Foundation. pp. 230–. ISBN 978-0-941901-21-5.
- Nelson, W. L., Wennerstrom, J. E., Dyer, D. C., Engel, M. (July 1977). "Absolute configuration of glycerol derivatives. 4. Synthesis and pharmacological activity of chiral 2-alkylaminomethylbenzodioxans, competitive .alpha.-adrenergic antagonists". Journal of Medicinal Chemistry. 20 (7): 880–885. doi:10.1021/jm00217a002. eISSN 1520-4804. ISSN 0022-2623.
- Fourneau Ernest, U.S. Patent 2,056,046 (1936 to Rhone Poulenc Sa).