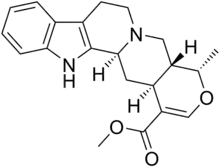
Ajmalicine
Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure.[1] It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan.[1] It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.[1][2][3]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.900 |
| Chemical and physical data | |
| Formula | C21H24N2O3 |
| Molar mass | 352.434 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 262.5 to 263 °C (504.5 to 505.4 °F) |
SMILES
| |
InChI
| |
| | |
Ajmalicine is structurally related to yohimbine, rauwolscine, and other yohimban derivatives. Like corynanthine, it acts as a α1-adrenergic receptor antagonist with preferential actions over α2-adrenergic receptors, underlying its hypotensive rather than hypertensive effects.[1][4]
Additionally, it is a very strong inhibitor of the CYP2D6 liver enzyme, which is responsible for the breakdown of many drugs. Its binding affinity at this receptor is 3.30 nM.[5]
See also
- Corynanthine
- Rauwolscine
- Spegatrine
- Yohimbine
References
- Wink, Michael; Roberts, M. W. (1998). Alkaloids: biochemistry, ecology, and medicinal applications. New York: Plenum Press. ISBN 0-306-45465-3.
- Kurz WG, Chatson KB, Constabel F, et al. (May 1981). "Alkaloid Production in Catharanthus roseus Cell Cultures VIII1". Planta Medica. 42 (5): 22–31. doi:10.1055/s-2007-971541. PMID 17401876.
- León F, Habib E, Adkins JE, Furr EB, McCurdy CR, Cutler SJ (July 2009). "Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A". Natural Product Communications. 4 (7): 907–10. doi:10.1177/1934578X0900400705. PMID 19731590. S2CID 37709142.
- Roquebert J, Demichel P (October 1984). "Inhibition of the alpha 1 and alpha 2-adrenoceptor-mediated pressor response in pithed rats by raubasine, tetrahydroalstonine and akuammigine". European Journal of Pharmacology. 106 (1): 203–5. doi:10.1016/0014-2999(84)90698-8. PMID 6099269.
- Strobl GR, von Kruedener S, Stöckigt J, Guengerich FP, Wolff T (1993). "Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modeling and inhibition studies". J Med Chem. 36 (9): 1136–45. doi:10.1021/jm00061a004. PMID 8487254.
{{cite journal}}: CS1 maint: multiple names: authors list (link)