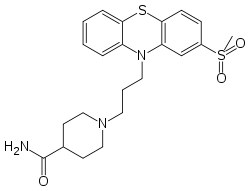
Metopimazine
Metopimazine (INN, USAN, BAN), sold under the brand names Nortrip, Vogalen, and Vogalene, is an antiemetic of the phenothiazine group which is used to treat nausea and vomiting.[1][2][3][4][5][6] It is marketed in Europe, Canada, and South America.[2][5] As of August 2020, metopimazine has been repurposed and is additionally under development for use in the United States for the treatment of gastroparesis.[6][5]
 | |
| Clinical data | |
|---|---|
| Trade names | Nortrip, Vogalen, Vogalene |
| Other names | EXP-999; NG-102; RP-9965 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.367 |
| Chemical and physical data | |
| Formula | C22H27N3O3S2 |
| Molar mass | 445.60 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Metopimazine has antidopaminergic, antihistamine, and anticholinergic activity.[7] However, it has also been described as a highly potent and selective dopamine D2 and D3 receptor antagonist.[5] The D2 receptor antagonism of metopimazine is thought to underlie its antiemetic and gastroprokinetic effects.[5] It is said to not readily cross the blood–brain barrier and hence to have peripheral selectivity, in contrast to metoclopramide but similarly to domperidone.[5] Unlike domperidone however, metopimazine shows no hERG inhibition and hence is expected to have a more favorable cardiovascular profile.[5] In contrast to metoclopramide, metopimazine does not interact with serotonin 5-HT3 and 5-HT4 receptors.[5]
Synthesis
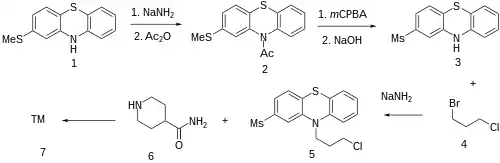
For the first step, 2-Methylthiophenothiazine [7643-08-5] (1) is protected by sequential reaction with sodium amide and acetic anhydride to give 1-[2-(Methylthio)-10H-phenothiazin-10-yl]ethanone [23503-69-7] (2). Oxidation with peracid proceeds preferentially on the more electron-rich alkyl thioether to give the sulfone. Upon hydrolysis of the acetate this affords 2-(methylsulfonyl)-10h-phenothiazine [23503-68-6] (3). Alkylation with 1-Bromo-3-chloropropane (4) gives 10-(3-chloropropyl)-2-methylsulfonylphenothiazine [40051-30-7] (5). Alkylation with piperidine-4-carboxamide (Isonipecotamide) [39546-32-2] (6) affords metopimazine (7).
References
- J. Elks, ed. (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 817–. ISBN 978-1-4757-2085-3. OCLC 1058412474.
- Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society (ed.). Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 683–. ISBN 978-3-88763-075-1.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 180–. ISBN 9789401144391. OCLC 1243535030.
- Herrstedt J (September 1998). "Chemotherapy-induced nausea and vomiting with special emphasis on metopimazine". Danish Medical Bulletin. 45 (4): 412–22. PMID 9777292.
- Heckroth M, Luckett RT, Moser C, Parajuli D, Abell TL (April 2021). "Nausea and Vomiting in 2021: A Comprehensive Update". J Clin Gastroenterol. 55 (4): 279–299. doi:10.1097/MCG.0000000000001485. PMC 7933092. PMID 33471485.
- "Metopimazine - Neurogastrx - AdisInsight".
- Bezin J, Noize P, Mansiaux Y, Jarne A, Pariente A (March 2021). "Antidopaminergic antiemetics and trauma-related hospitalization: A population-based self-controlled case series study". Br J Clin Pharmacol. 87 (3): 1303–1309. doi:10.1111/bcp.14510. ISSN 0306-5251. PMID 32737898. S2CID 220909387.
- Robert Michel Jacob, Jacques Georges Robert, DE 1092476 (1960 to Rhone Poulenc Sa).
- Karicherla, Venumanikanta; Phani, Kumar; Bodireddy, Mohan Reddy; Prashanth, Kumar Babu; Gajula, Madhusudana Rao; Pramod, Kumar (2017). "A Simple and Commercially Viable Process for Improved Yields of Metopimazine, a Dopamine D2-Receptor Antagonist". Organic Process Research & Development. 21 (5): 720–731. doi:10.1021/acs.oprd.7b00052.